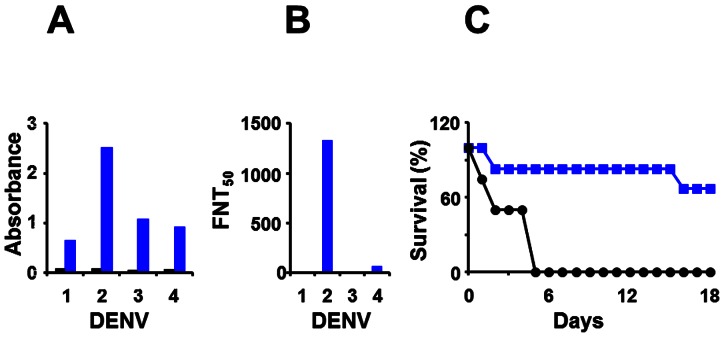
Figure 6. Characterization of DENV-2-specific antibodies elicited by DENV-2 E VLPs.
(A) Analysis of virus-specific antibody titers in anti-DENV-2 E antisera (blue bars) and mock-immune sera (black bars) in indirect ELISAs using infectious DENVs as coating antigen. (B) Determination of virus-neutralizing antibody titers using FACS neutralization assay. Serial dilutions of anti-DENV-2 E antisera were tested for their capacity to neutralize infectivity of all four DENV serotypes [30]. The vertical axis denotes the serum dilution corresponding to 50% neutralization (FNT50 titre) of virus infectivity. Murine sera used in experiments shown in panels A and B were from Balb/C mice; the Arabic numerals along the x-axis, in both these panels, indicate DENV serotype. (C) Determination of protective efficacy of DENV-2 E VLP immunization. AG129 mice were either mock-immunized (black curve, n = 4) or immunized with DENV-2 E VLPs (blue curve, n = 6) and challenged with a virulent strain of DENV-2. The mice were monitored daily (up to 18 days post challenge) for mortality and the resultant data plotted as Kaplan-Meir survival curves.