Abstract
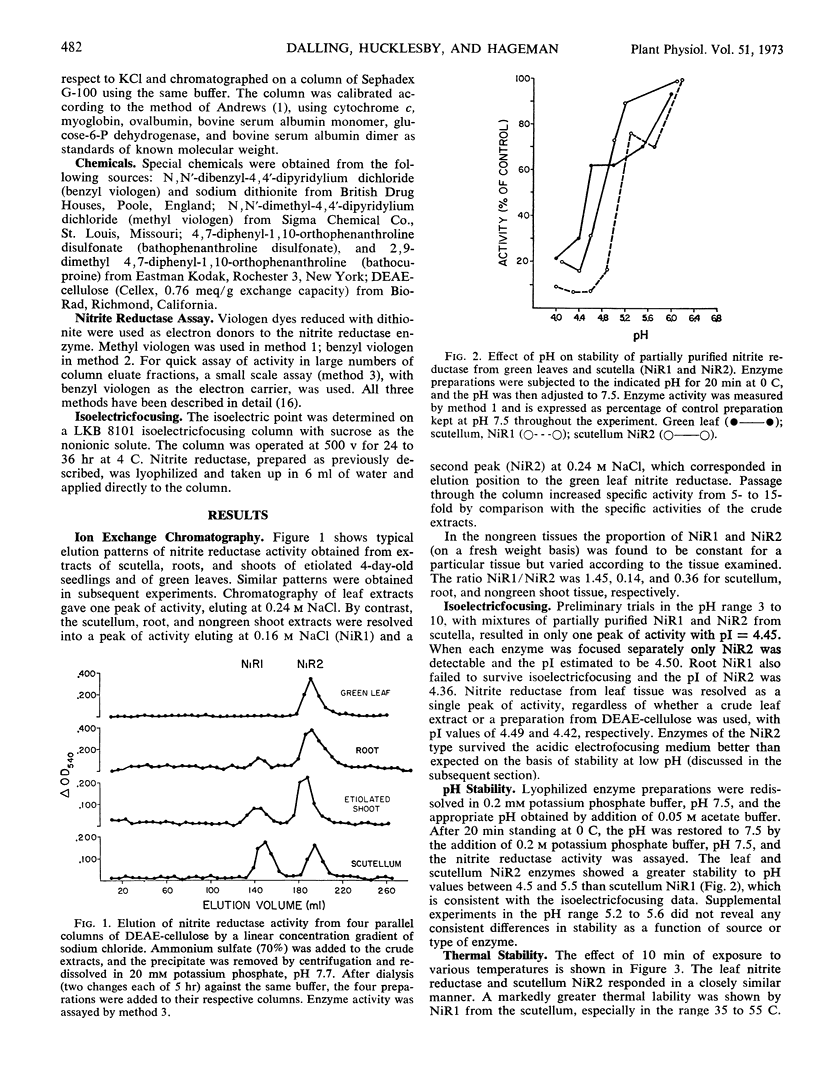
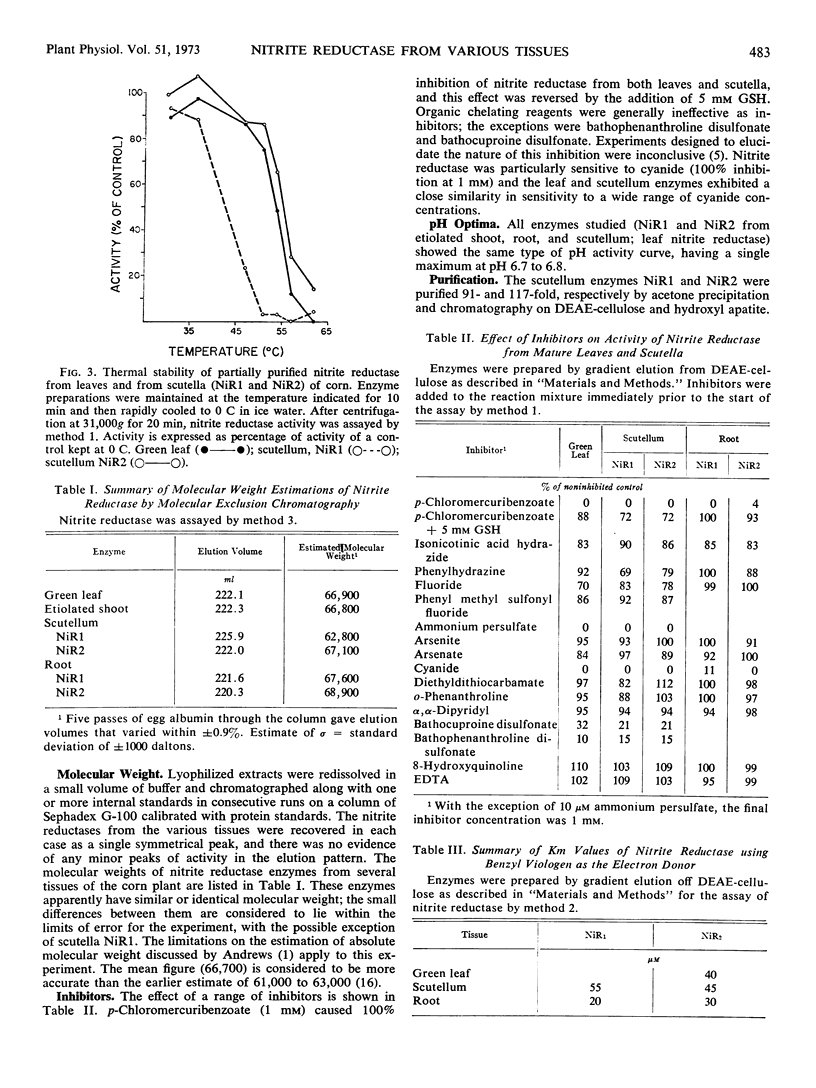
Nitrite reductase from green leaves of corn (Zea mays L.) is eluted from a diethylaminoethyl-cellulose column in one peak of activity by a chloride gradient, while nitrite reductase from scutellum tissue is resolved into two peaks of activity, apparently representing two forms of the enzyme NiR1 and NiR2. One of these (NiR2) elutes at the same concentration of chloride as the leaf nitrite reductase. Roots and etiolated shoots also exhibited both forms of the enzyme, however, lesser amounts of NiR1 is extractable from these tissues than from scutellum. Comparison of green leaf nitrite reductase with NiR2 from scutellum tissue shows similar or identical properties with respect to molecular weight, isoelectric point, electron donor requirements, inhibition properties, pH optima, thermal stability, and pH tolerance. The significance of these similarities in relation to probable differences in the biochemical mechanism of nitrite reduction between leaf and scutellum tissues is discussed. Although ferredoxin is considered, with some reservations, to be the electron donor for nitrite reductase in green tissue, the reductant for nongreen tissue is not known. The possibility that nitrite reductases from green and non-green tissues uses the same electron donor, in vivo, is considered.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott J. P., Lominski I. R., Wright M. R. Inhibition of staphylococcal alpha-toxin. The effect of aromatic polysulphonic acids on the lethal effect of alpha-toxin in mice. Biochem J. 1968 Jun;108(1):49–55. doi: 10.1042/bj1080049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTT V. S., BEEVERS H. The regulation of pathways of glucose catabolism in maize roots. Biochem J. 1961 Jul;80:21–27. doi: 10.1042/bj0800021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne W. F., Miflin B. J. An ATP dependent reduction of nitrate to ammonia by a cell free particulate system from barley roots. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1305–1310. doi: 10.1016/0006-291x(70)90008-2. [DOI] [PubMed] [Google Scholar]
- CRESSWELL C. F., HAGEMAN R. H., HEWITT E. J., HUCKLESBY D. P. THE REDUCTION OF NITRATE, NITRITE AND HYDROXYLAMINE TO AMMONIA BY ENZYMES FROM CUCURBITA PEPO L. IN THE PRESENCE OF REDUCED BENZYL VIOLOGEN AS ELECTRON DONOR. Biochem J. 1965 Jan;94:40–53. doi: 10.1042/bj0940040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Varner J. E. Control of nitrate reductase activity in barley aleurone layers. Proc Natl Acad Sci U S A. 1970 Mar;65(3):729–736. doi: 10.1073/pnas.65.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Varner J. E. Intact tissue assay for nitrite reductase in barley aleurone layers. Plant Physiol. 1971 Jun;47(6):790–794. doi: 10.1104/pp.47.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucklesby D. P., Hewitt E. J. Nitrite and hydroxylamine reduction in higher plants. Fractionation, electron donor and substrate specificity of leaf enzymes, principally from vegetable marrow (Cucurbita pepo L.). Biochem J. 1970 Oct;119(4):615–627. doi: 10.1042/bj1190615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W., Hageman R. H. The purification and properties of nitrite reductase from higher plants, and its dependence on ferredoxin. Biochem J. 1966 Jul;100(1):263–273. doi: 10.1042/bj1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP J. D., ATKINSON D. E., EHRET A., LAZZARINI R. A. EVIDENCE FOR THE IDENTITY OF THE NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-SPECIFIC SULFITE AND NITRITE REDUCTASES OF ESCHERICHIA COLI. J Biol Chem. 1963 Oct;238:3466–3471. [PubMed] [Google Scholar]
- MEDINA A., NICHOLAS D. J. Metallo-enzymes in the reduction of nitrite to ammonia in Neurospora. Biochim Biophys Acta. 1957 Jul;25(1):138–141. doi: 10.1016/0006-3002(57)90430-4. [DOI] [PubMed] [Google Scholar]
- Ritenour G. L., Joy K. W., Bunning J., Hageman R. H. Intracellular localization of nitrate reductase, nitrite reductase, and glutamic Acid dehydrogenase in green leaf tissue. Plant Physiol. 1967 Feb;42(2):233–237. doi: 10.1104/pp.42.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. II. Reduction of Nitrite to Ammonia. Plant Physiol. 1964 May;39(3):423–431. doi: 10.1104/pp.39.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swader J. A., Stocking C. R. Nitrate and Nitrite Reduction by Wolffia arrhiza. Plant Physiol. 1971 Feb;47(2):189–191. doi: 10.1104/pp.47.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]



