Figure 1.
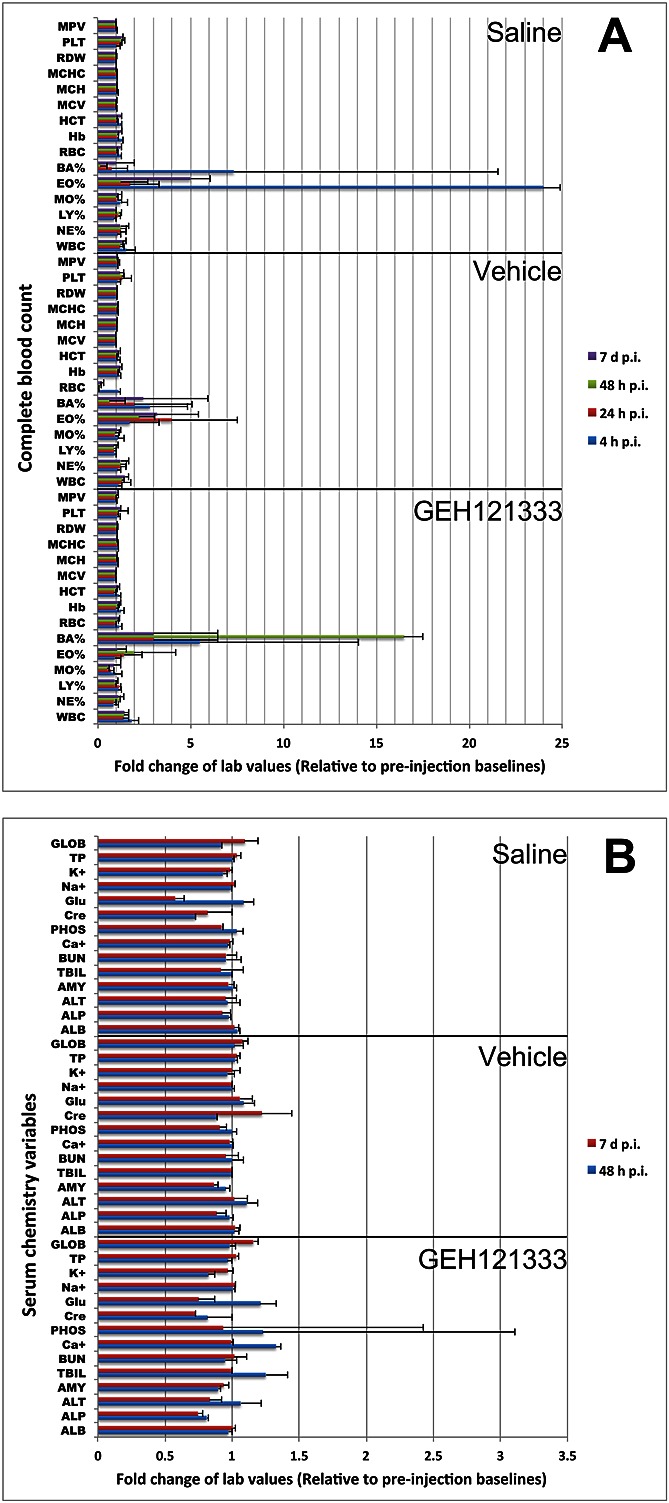
No clinically significant changes in complete blood count and serum chemistry after high dose GEH121333 injection. Intravenous injection of GEH121333 at 200 mg Fe kg−1 did not generate clinically significant alteration in complete blood count (A) and serum chemistry (B) profiles up to 7 days p.i. compared with laboratory values of vehicle or saline-treated animals. MPV, mean platelet volume; PLT, platelet count; RDW, red blood cell distribution width; MCHC, mean corpuscular hemoglobin concentration; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; HCT, hematocrit; Hb, hemoglobin; RBC, red blood cell count; BA%, percentage basophil count; EO%, percentage eosinophil count; MO%, percentage monocyte count; LY%, percentage lymphocyte count; NE%, percentage neutrophil count; WBC, white blood cell count; GLOB, globulin; TP, total protein; K+, potassium; Na+, sodium; Glu, glucose; Cre, creatinine; PHOS, phosphate; Ca+, calcium; BUN, blood urea nitrogen; TBIL, total bilirubin; AMY, amylase; ALT, alanine transaminase; ALP, alkaline phosphatase; ALB, albumin.
