Figure 1.
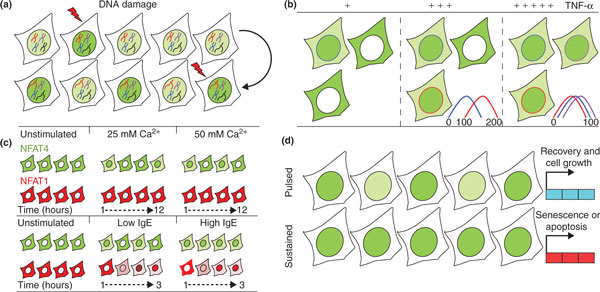
Transcription factor dynamics in single living cells. (a) DNA damage affects transcription factor dynamics. p53 (green) exhibits more frequent nuclear pulses as damage levels increase (disrupted black and red chromosomes). (b) Increasing doses of extracellular stimulation (plus signs) affects the synchronization of the initial cytoplasm-to-nucleus translocation time. High levels of TNF-α increase the overall population response, observed as elevated nuclear NF-κB (green) and increased synchronization. Colored plots represent the initial NF-κB nuclear translocation times (minutes) for each depicted cell. (c) NFAT isoforms respond differently to stimuli. Top: calcium levels modulate the frequency of NFAT4 nuclear localization. A rise in calcium levels increases the frequency of NFAT4 nuclear localization (green) bursts, which last for up to 12 hours, while NFAT1 remains cytoplasmic (red). Bottom: immunoglobulin E (IgE) mediates activation of NFAT1 and NFAT4. In response to low antigen levels, NFAT4 exhibits nuclear bursts that become sustained nuclear localization at high antigen levels. NFAT1 shows a gradual response to both low and high antigen levels, but with higher amplitude at high levels. (d) Unique transcription factor dynamics control gene expression patterns. Pulsed or sustained p53 nuclear accumulation (green) under DNA damage conditions determines cell fate by driving expression of distinct sets of genes. Pulsed p53 leads to cell recovery and proliferation, whereas sustained p53 ends in activation of senescence or apoptosis pathways.
