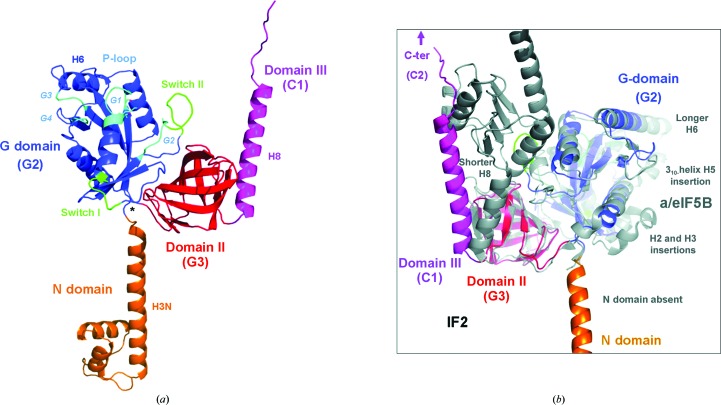
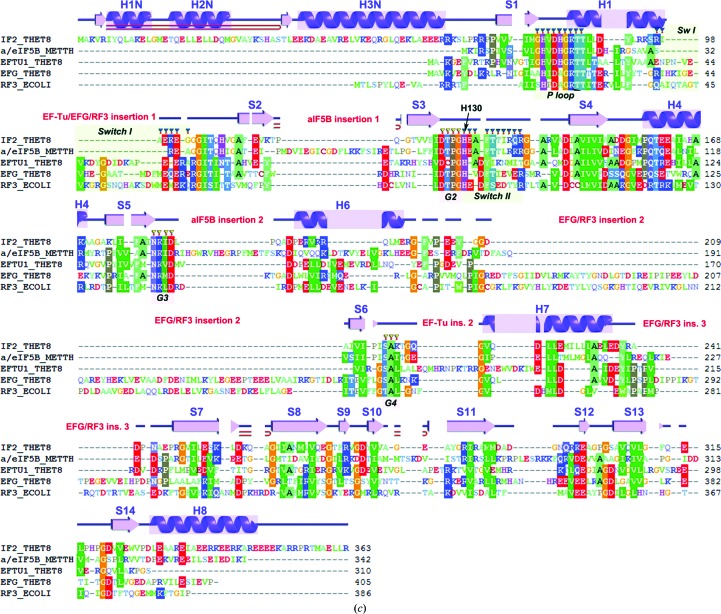
Figure 1.
Crystal structure of IF2. (a) Overall structure of T. thermophilus IF2 (residues 1–363). Domains are annotated and colour-coded: N domain, orange; G domain, blue; domain II, red; beginning of domain III, pink. The switch I and II regions are indicated in green and the nucleotide-binding motifs G1 (P-loop), G2, G3 and G4 are highlighted in cyan. Helix H3N from the N domain and helix H8 from domain III are indicated. (b) Comparison of the T. thermophilus IF2 (this work) and M. thermoautotrophicum a/eIF5B (PDB entry 1g7t, GDPNP complex; Roll-Mecak et al., 2000 ▶) structures showing significant structural differences between the eubacterial and archaeal homologues [IF2 domains are colour-coded as in (a); the view focuses on the structurally conserved core comprising the G domain and domain II; the view is rotated 180° compared with (a) in order to better illustrate the structural differences; the position of the C-terminal region beyond residue 363 of IF2 is indicated by an arrow]. (c) Structure-based sequence alignment of T. thermophilus IF2 with related proteins (archaeal a/eIF5B from M. thermoautotrophicum, T. thermophilus EF-Tu, T. thermophilus EF-G and E. coli RF3). The G1, G2, G3 and G4 motifs that are in the vicinity of the nucleotide are annotated, as well as the switch I and switch II regions.