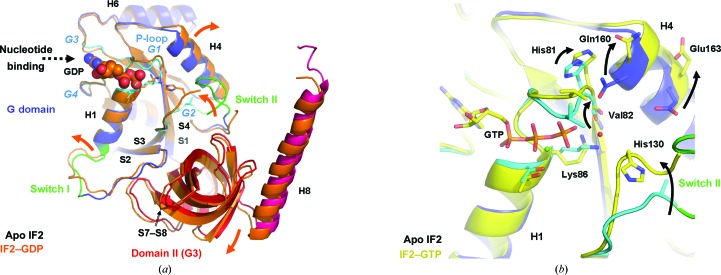
Figure 2.
Conformational changes of IF2 upon nucleotide binding. (a) The superposition of the apo IF2 (colour-coded as in Fig. 1 ▶ a) and the IF2–GDP complex (in orange) shows the overall conformational changes of IF2 that occur upon nucleotide binding (highlighted by arrows; important secondary-structure elements are annotated as in Fig. 1 ▶; for simplicity, the N domain is not shown). (b) Detailed view of the nucleotide-dependent conformational changes, revealing the key role of Val82 in the P-loop that transmits conformational changes to Gln160 of helix H4 and the concomitant conformational change of the switch II region (apo IF2 colour-coded as in Fig. 1a and IF2–GTP in yellow). The transmission of conformational changes occurs at the cross-over of two loops (marked with an asterisk in Fig. 1 ▶ a) that connect the ends of the G domain to the neighbouring domains, suggesting that this region could act as a node coordinating nucleotide-dependent domain movements.