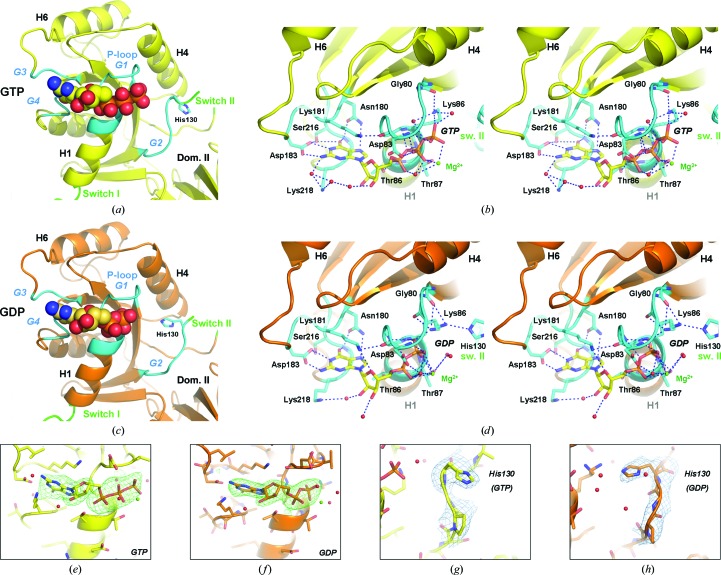
Figure 3.
Molecular recognition and GTP/GDP-dependent conformational changes in the catalytic site of IF2. (a, c) Overall binding site of the nucleotides as observed in the IF2–GTP and IF2–GDP complexes. The nucleotide is inserted in the P-loop (G1 motif) and between the G2, G3 and G4 motifs (in cyan; see sequence alignment in Fig. 1 ▶ c); switches I and II are indicated in green. (b, d) Detailed hydrogen-bond pattern of the IF2–GTP and IF2–GDP complexes (stereo representations). Amino-acid residues interacting via their polypeptide backbone or their side chains are indicated; hydrogen bonds are indicated by dotted lines. The guanosine moiety of the nucleotide interacts mostly with the G3 and G4 motifs and the ribose moiety shows hydrogen bonds to Lys218 mediated through water molecules (red spheres), while the phosphate groups interact with residues from the P-loop. Lys181 helps to stabilize the G3 loop with the P-loop. The Mg2+ ion (in green) interacts with the β- and γ-phosphate moieties of the GTP, and with the GDP α- and β-phosphate moieties and with Thr87 in the IF2–GDP complex. The position of the Mg2+ ion is almost identical in both complexes, while the β-phosphate moiety is rotated. An octahedral coordination of the Mg2+ ion is not observed, probably because the switch I region is much shorter than in translation elongation factors; additional coordination may potentially be provided by the 50S ribosomal subunit when IF2 is ribosome-bound. Lys86 (P-loop) interacts with the terminal phosphate group of the nucleotide and changes conformation between the GTP-bound and GDP-bound states, while His130 interacts with Lys86 specifically in the GDP complex. In the GTP complex a water molecule is positioned next to the γ-phosphate. The side chain of His130 is flipped out in the GTP-bound state (not visible in this view) and flipped in in the GDP-bound state. Leu184 is not shown for simplicity (it would overlap with Asp183). (e, f) OMIT maps of the GTP and GDP complexes shown together with the final refined structures. (g, h) Electron density of the His130 region for the GTP–IF2 and GDP–IF2 complexes (shown for residues 128–130 for simplicity), providing experimental evidence for the conformational change of His130 and the switch II loop.