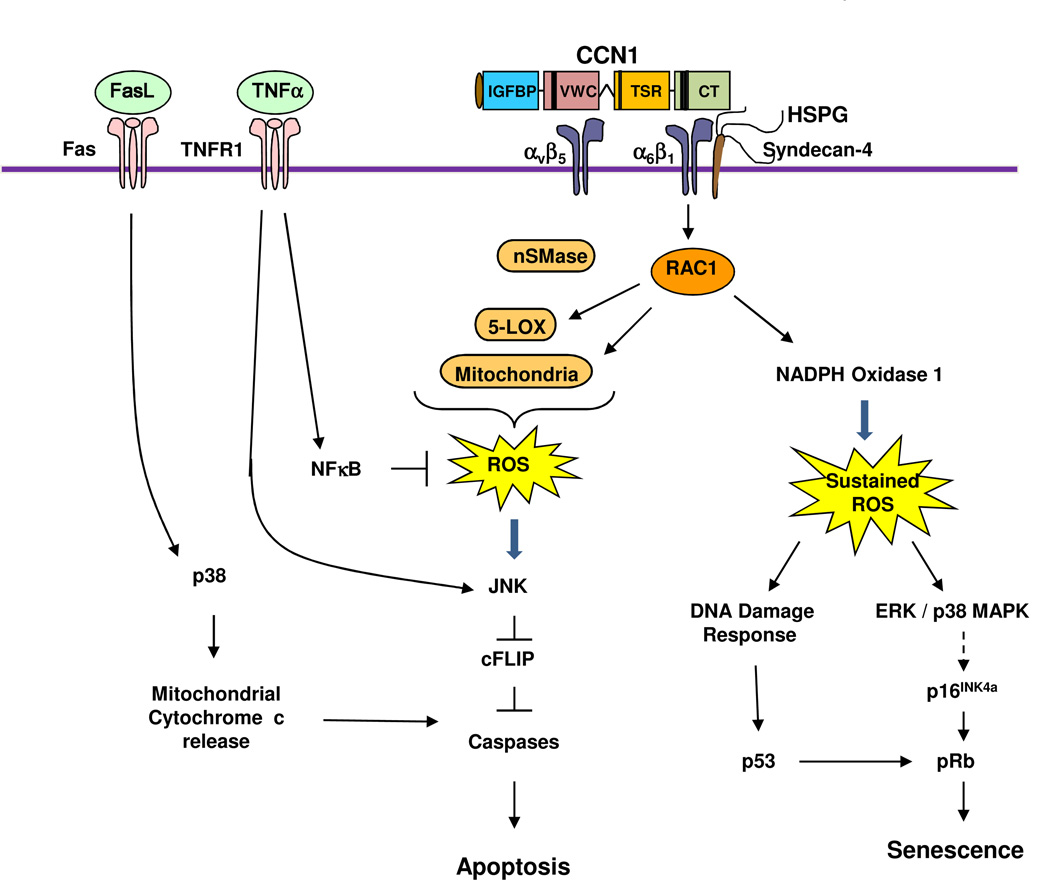
Figure 3. Signaling mechanism of CCN1-induced senescence and crosstalk with TNFα and FasL.
The binding of CCN1 to integrin α6β1-HSPGs (syndecan 4) triggers the activation of RAC-1 and NADPH oxidase 1, leading to a much more robust and sustained level of ROS compared to cell adhesion to other ECM proteins. The sustained ROS induces a DNA damage response and the activation of p53, and triggers the activation of ERK and p38 MAPK, which in turn induces p16INK4a and activates pRb8. Activated p53 and pRb contribute to the induction of cellular senescence. If integrin αvβ5 is also engaged by CCN1, RAC1-dependent ROS accumulation includes contribution from 5-lipoxygenase (5-LOX) and the mitochondria7; neutral sphingomyelinase (nSMase) also contributes to CCN1-induced ROS26. CCN1-induced ROS counteracts the effect of NFκB, which is strongly activated by TNFα and induces the expression of antioxidant proteins. The high level of ROS inhibits cysteine phosphatases that can inactivate MAPKs such as JNK, ERK, and p38 MAPK, leading to a hyperactivation of these kinases219. Activated JNK targets the proteosomal degradation of cFLIP220, an inhibitor of caspase activation, allowing the activation of caspases 8/10 by TNFα to induce apoptosis without blocking de novo protein synthesis or NFκB signaling7. In addition to CCN1, CCN2 and CCN3 also synergize with TNFα to induce apoptosis, presumably through a similar mechanism25. In the presence of FasL, which can trigger apoptosis on its own, CCN1 or CCN2-induced ROS leads to the hyperactivation of p38 MAPK, which enhances cytochrome c release from the mitochondria and thereby increases apoptosis26.