Abstract
The healing process after any surgical intervention has always posed a challenge for the surgeons. In spite of the advances in wound closure techniques and devices, there is a crucial need for newer methods of enhancing the healing process to achieve optimal outcomes. Fibrin adhesives and platelet concentrates have proven useful in various treatment modalities in the fields of microvascular and plastic surgery. This case report shows its unique use in the field of maxillofacial and cutaneous surgery. It shows an innovative technique of enhancement of skin wound healing by local application of platelet-rich fibrin.
KEYWORDS: Biologic dressing, modified secondary intention healing, platelet-rich fibrin
INTRODUCTION
Despite the modern advances in wound closure techniques and devices, there is a vital need for newer methods of enhancing the healing process to achieve optimal outcomes. Wound healing is a complex sequence of intracellular and extracellular events regulated by signaling proteins. This process is, at present, not completely understood. One of the promising, but complicated areas of recent therapeutic development involves topical application of growth factors to enhance the normal healing process. This case report shows an innovative technique of enhancement of healing of skin wounds by local application of platelet-rich fibrin (PRF) as a biological dressing.
CASE REPORT
A 30-year-old man who suffered a motorcycle accident, reported to the Department of Oral and Maxillofacial Surgery, presenting with an avulsive wound, approximately 0.5 × 1 cm in size, on the lower lip in the midline region with a disruption of the vermilion border and a white roll [Figure 1]. The depth of the wound was uneven with muscle tissue loss in some regions. The patient had no medical history. Primary closure could have caused pouting of the lip. Healing by secondary intention could have resulted in fibrosis and development of a hideous scar. Therefore, it was decided to treat the defect by local application of a PRF membrane.
Figure 1.
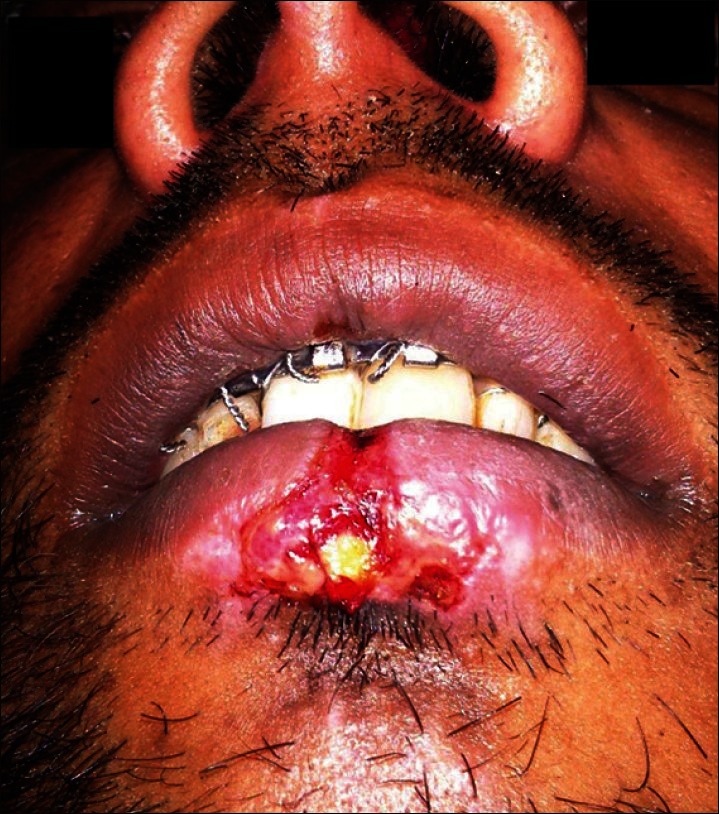
Avulsive wound over the lower lip
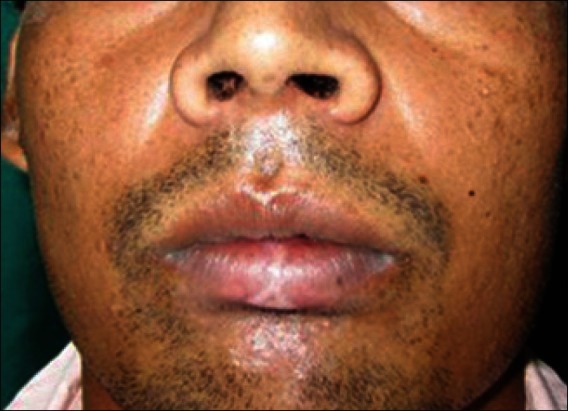
About 10 ml of venous blood was withdrawn to prepare PRF by Choukroun's protocol [Figure 2].[1] The sampled blood was transferred into glass test tubes. It was then centrifuged at 3000 rpm for 10 min in a laboratory centrifuge. At the end of the centrifugation, three distinct layers could be seen in the test tube. The uppermost layer was platelet poor plasma, the middle layer was platelet rich fibrin, and the lowermost layer was red blood cells. The middle layer of PRF was separated and squeezed between two layers of sterile gauze. The PRF membrane thus obtained was placed over the defect [Figure 3]. A double layer of the membrane was placed over deeper areas of the defect to achieve an even surface. Neglecting this step could lead to an uneven surface and formation of a scar on healing; this was covered by a single layer of moist gauze and micropore dressing. A gradual decrease in the size of the wound and granulation tissue formation was observed in the subsequent visits [Figure 4]. There were no signs of inflammation or infection. It was observed that the PRF-treated site showed hastened healing with early wound contracture. In the subsequent visits, the color of the reconstructed region was comparable to the adjacent uninjured tissue. The defect in the vermilion border and the white roll was inconspicuous [Figure 5].
Figure 2.
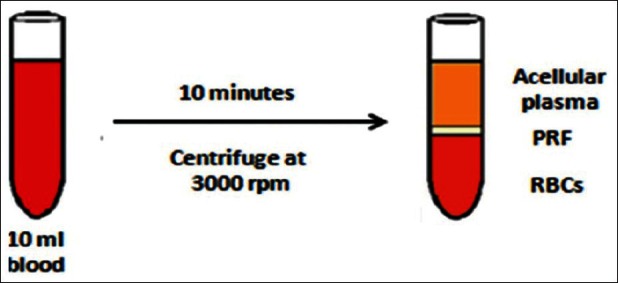
Processing of platelet-rich fibrin
Figure 3.
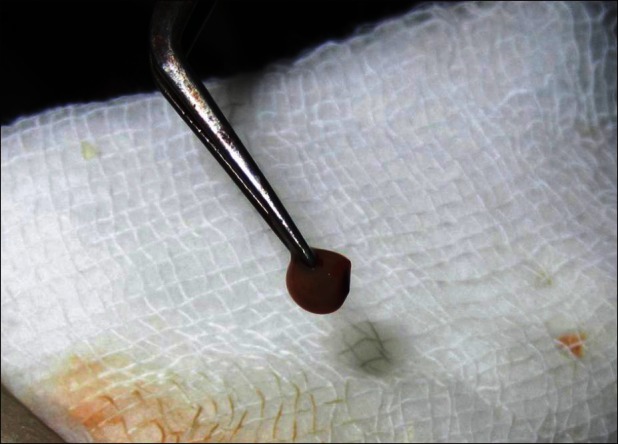
Platelet-rich fibrin membrane
Figure 4.
Two weeks follow up
Figure 5.
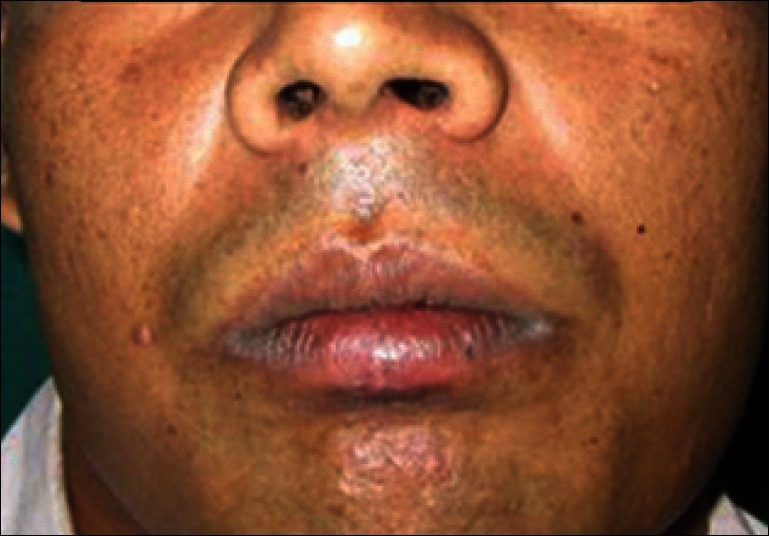
Six weeks follow up
DISCUSSION
Face being a highly aesthetic zone, the complexities in healing after any intervention poses a great challenge to the surgeon. Scar formation is one of the most undesirable outcomes of the healing process. Early primary closure helps minimize scars, but in cases where there is skin loss, it is not always achievable. This is true, especially in areas where undermining the adjacent tissue is not possible or it may distort facial symmetry.
It is certain that platelets play a prominent, if not deciding role, in wound healing.[2] Platelets release cytokines and growth factors during clot formation at the wound sites.[3] Platelet activation in response to tissue damage and vascular exposure results in the formation of a platelet plug and blood clot to provide hemostasis and the secretion of biologically active proteins from the α-granules. These include platelet derived growth factor (PDGF), transforming growth factor (TGF), platelet factor 4 (PF4), interleukin (IL)-1, vascular endothelial growth factor (VEGF), platelet-derived angiogenesis factor (PDAF), epidermal growth factor (EGF), insulin-like growth factor (IGF), epithelial cell growth factor (ECGF), osteonectin, osteocalcin, fibrinogen, vitronectin, fibronectin, and thromboplastin.[2] These secretory proteins, in turn, initiate tissue healing. Healing includes cellular chemotaxis, proliferation, and differentiation and the removal of tissue debris, angiogenesis, laying down of extracellular matrix, and regeneration of the appropriate type of tissue. Platelets direct wound healing because, by design, they appear exactly where and when needed to create a local environment conducive to tissue regeneration and set the pace of wound healing with their effects lasting even after the clot has been cleared. The improvement of healing by the placement of a supraphysiologic concentration of autologous platelets at the site of tissue injury is supported by basic science studies. There are studies that provide evidence that the use of autologous platelet-rich plasma offers some efficacy in certain types of acute and chronic wounds and causes an increase in hard- and soft-tissue wound healing and a decrease in postoperative infection, pain, and blood loss.[2]
PRF is a second generation platelet concentrate with simplified processing without biochemical blood handling. It enmeshes glycosaminoglycans from the blood and platelets, which have a strong affinity with small circulating peptides and a great capacity to support cell migrations and healing processes. Dohan et al. (2006) stated that PRF was a completely usable healing concentrate.[1]
Hom et al. (2007) in a study on the healing effects of autologous platelet gel (APG) on acute human skin wounds concluded that APG not only enhanced wound closure but also increased the wound healing velocity.[4]
Role of platelet-rich fibrin membrane in healing
Any avulsive wound gets covered with a blood clot. This clot is the focal point of initiating healing. A natural blood clot contains 5% platelets, 95% red blood cells, <1% white blood cells, and large amounts of fibrin strands. A platelet-rich plasma (PRP) clot contains 95% platelets, 4% red blood cells, and 1% white blood cells. PRP is a first generation plasma concentrate.[5] Due to the various advantages of PRF over PRP and the ease of technique, PRF is preferred over the latter. PRF membrane is a simple approach of concentrating platelets or enriching natural blood clot, which forms in avulsive wounds, to initiate a more rapid and complete healing process.
PRF is an immune and platelet concentrate that collects on a single fibrin membrane; all the constituents of a blood sample are favorable to healing and immunity.[6] Although platelet and leukocyte cytokines are important in the biology of this biomaterial, the fibrin matrix supporting them constitutes the determining element responsible for the real therapeutic potential of PRF. The PRF membranes simultaneously support the development of the three keys to healing and soft tissue maturation, namely, angiogenesis, immunity, and epithelial cover. This membrane protects open wounds and accelerates healing, favors the development of microvascularization, and also guides epithelial cell migration to its surface. Furthermore, this matrix contains leukocytes and promotes their migration. Therefore, its utilization seems to be of high significance in the case of infected wounds, like that of rapid uneventful healing of a tooth socket filled with PRF.[6]
Since the healing that occurs by using this technique is neither by primary intention nor by pure secondary intention, we have termed it “modified secondary intention healing.” The efficacy of this treatment lies in the local delivery of a wide range of growth factors and proteins on a fibrin mesh, mimicking and supporting physiological wound healing and reparative tissue processes, thereby resulting in a nearly scarless aesthetic wound healing.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–59e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 3.Blanton MW, Hadad I, Johnstone BH, Mund JA, Rogers PI, Eppley BL, et al. Adipose stromal cells and platelet-rich plasma therapies synergistically increase revascularization during wound healing. Plast Reconstr Surg. 2009;123:56S–64S. doi: 10.1097/PRS.0b013e318191be2d. [DOI] [PubMed] [Google Scholar]
- 4.Hom DB, Linzie BM, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174–83. doi: 10.1001/archfaci.9.3.174. [DOI] [PubMed] [Google Scholar]
- 5.Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: Evolution of a second generation platelet concentrate. Indian J Dent Res. 2008;19:42–6. doi: 10.4103/0970-9290.38931. [DOI] [PubMed] [Google Scholar]
- 6.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56–60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]


