Abstract
Timely diagnosis of HIV is essential to improve survival rates and reduce transmission of the virus. Insufficient progress has been made in effecting earlier HIV diagnoses. The Mexican border city of Tijuana has one of the highest AIDS incidence and mortality rates in all of Mexico. This study examined the prevalence and potential correlates of late HIV testing in Tijuana, Mexico. Late testers were defined as participants who had at least one of: (1) an AIDS-defining illness within 1 year of first positive HIV test; (2) a date of AIDS diagnosis within 1 year of first positive HIV test; or (3) an initial CD4 cell count below 200 cells per microliter within 1 year of first positive HIV test. Medical charts of 670 HIV-positive patients from two HIV/AIDS public clinics in Tijuana were reviewed and abstracted; 362 of these patients were interviewed using a cross-sectional survey. Using multivariate logistic regression, we explored potential correlates of late HIV testing based on the Behavioral Ecological Model. From 342 participants for whom late testing could be determined, the prevalence of late testing was 43.2%. Multivariate logistic regression results (n = 275) revealed five significant correlates of late testing: “I preferred not to know I had HIV” (adjusted odds ratio [AOR] = 2.78, 1.46–5.31); clinic (AOR = 1.90, 1.06–3.41); exposure to peers engaging in high-risk sexual behavior (AOR = 1.14, 1.02–1.27); stigma regarding HIV-infected individuals (AOR = 0.65, 0.47–0.92); and stigma regarding HIV testing (AOR = 0.66, 0.45–0.97). These findings may inform the design of interventions to increase timely HIV testing and help reduce HIV transmission in the community at large.
Introduction
Timely diagnosis of HIV infection is essential to decrease morbidity and mortality among those infected as well as to reduce transmission. Prompt identification of HIV and treatment with highly active antiretroviral therapy (HAART) may respectively reduce HIV transmission through risk behavior change and reduction of infectivity.1 Despite the benefits of timely identification of HIV infection, little or no progress has been made in effecting earlier diagnoses.2–7
Mexico is a country with an HIV prevalence of 0.3%,8 which is lower than its neighboring countries (i.e., Belize, 2.0%; Guatemala, 1.0%; Honduras, 1.6%; United States, 0.6%).9 However, HIV/AIDS rates vary widely across the country. Baja California presents with the second highest AIDS incidence rate in all of Mexico10 and has also maintained one of the highest AIDS mortality rates among Mexico's 31 states (9.2 deaths per 100,000 versus 4.7 per 100,000 nationwide).11
Fifty percent of Baja California's population resides in Tijuana,12 just south of San Diego, California. Anecdotal information from Tijuana health professionals suggests that many HIV-positive patients present undiagnosed with opportunistic infections and advanced AIDS disease without prior knowledge of HIV infection. Late HIV testing may contribute to the high AIDS incidence and mortality rates in Baja California, and may result in missed opportunities for preventive services and early medical care.
Previous studies in several countries have identified variables positively associated with late HIV testing or delayed diagnosis including: heterosexual transmission2–4,6,13,14; male gender4,5,7,15; both older2,4,5,7,16–19 and younger3,13,20 age; foreign-born status3–6,13; belonging to racial/ethnic minority populations14,20; residing in areas with lower AIDS incidence3,5; being less educated16,20; and having tested negative for HIV prior to the first positive HIV test.20
Whether these or other factors are correlated to late HIV testing in the San Diego-Tijuana region is unknown. While some of the factors associated with HIV testing have been studied using individual and interpersonal models of health behavior,21,22 the factors examined in this study were selected based on the Behavioral Ecological Model (BEM).23,24 The BEM is a hierarchical model of health-related behavior that stems from learning theory and emphasizes individual contingencies of reinforcement, meta-contingencies that take place among groups engaged in complex coordinated behaviors, and macrocontingencies that directly impact large groups or whole populations. Among the independent variables selected for inclusion as possible correlates of late HIV testing in the present analysis, we attempted to include variables representing each of these levels of contingencies (or markers of them). For example, we have incorporated measures of stigma related to persons with HIV infection and stigma related to HIV testing as markers of social prejudice, both presumed to motivate individuals to avoid HIV testing.
As the HIV/AIDS epidemic has evolved over the years to include cases from populations with diverse HIV risk profiles, potential correlates of testing late for HIV infection may be best explained through the BEM, as contrasted with primary and/or exclusive emphasis on individual characteristics and behaviors of the patient. The BEM has been previously applied to analyses of HIV risk reduction,25 physical activity,26 smoking bans,27 and weapon carrying.28 The purpose of this study was to examine the prevalence and potential correlates of late HIV testing among HIV-positive patients seeking care in Tijuana, Mexico, using a model based on the BEM.
Methods
Design
Medical charts for all available HIV-positive patients at two public clinics (N = 670) were abstracted for clinical and biological data. Cross-sectional interviews were conducted for as many of these patients as could be recruited (n = 362) in a 9-month period. The prevalence of late testing was determined for all possible interviewees (n = 342) and correlates of late HIV testing were explored (n = 275).
Recruitment and informed consent
Patients were recruited from the two public HIV/AIDS clinics in Tijuana, Mexico. These sites each provide care for over 300 HIV-infected patients per year, which represents nearly 90% of the persons receiving HIV/AIDS care in Tijuana. One clinic is affiliated with the Mexican Institute of Social Security (IMSS [Spanish acronym]), providing care for individuals with employer-based insurance. The second clinic is affiliated with the State's Ministry of Health (ISE Salud, [Spanish acronym]), providing free HIV/AIDS health care to the uninsured. All study procedures were approved by San Diego State University, IMSS, and ISE Salud Institutional Review Boards.
The sampling frame consisted of all HIV/AIDS patients seeking care at IMSS or ISE Salud. Patients diagnosed with HIV for at least 1 year were eligible to participate and were referred to study recruiters/interviewers by medical staff (clinics' receptionists and/or nurse practitioners) during a follow-up visit. Given the covert nature of HIV/AIDS in Tijuana, as well as clinic patient privacy policies and the ethical issues involved, members of the research team and clinic key personnel collaboratively arranged for medical staff to serve as intermediaries for study recruitment. The designated medical staff persons were asked and regularly reminded to invite all HIV/AIDS patients to participate in the study at the time of check-in for a follow-up visit, when physical measurements were taken, and/or when picking up prescriptions for medication refills.
Interested patients were referred to trained study recruiters/interviewers who provided detailed information about the study and obtained informed consent. Trained recruiters/interviewers were on site for recruitment and data collection on Wednesdays and Fridays at IMSS and on the other week days at ISE Salud, for approximately 4 h per day.
Patients who never attended the clinic during the recruitment period or had died (157; 23.4%), were too sick to participate (37; 5.5%), were unable to verbally communicate (7; 1.0%), were cognitively impaired (7; 1.0%), were imprisoned (9; 1.34%), were nonreferrals (23, 3.4%), missed follow-up visits (44; 6.6%), or refused to participate (24; 3.6%) were not recruited.
Participants
Of the 362 patients recruited and interviewed, 20 cases had to be excluded from analyses because of insufficient data to be classified as either late testers or non-late testers, leaving a sample size of 342. These participants were aged 18–72 years (mean 38.2, standard deviation [SD] = 9.9), and were 67.8% male. All participants, except for 2, were born in Mexico. Only 55 participants were born in Baja California Norte, the rest (83.9%) immigrated to Baja California Norte at some point in their lives. Most (93.9%) lived in Tijuana, 51.2% were employed, 66.3% were heads of household, and 14.1% of participants with children, had children who were also HIV positive. Approximately one fourth (26.6%) of participants had a history of residing in the United States in the past; of these individuals, 14.3% tested positive for the first time in the United States.
Medical chart abstraction
Data from all patients' medical records were abstracted by one of the physicians on the research team. To assess the interrater reliability of these data, a 10% random sample of charts was abstracted by an internal medicine resident affiliated with the Universidad Autonoma de Baja California in Tijuana. Percent agreement across all variables used for the present analysis ranged from 0.77 to 1.0. Of all the chart variables used, 91.8% had a percent agreement ≥0.91 and 55.7% had a percent agreement equal to 1.0. The variables obtained from medical charts were: age at HIV diagnosis; exposure category; date of first positive HIV test (obtained from laboratory report or physician documentation on the initial medical evaluation); date of AIDS diagnosis, evidence of AIDS-defining illnesses at HIV diagnosis and/or within 12 months of HIV diagnosis, initial CD4 cell count, and history of HAART. These variables were used for description and/or in the regression model.
Interview
Confidential interviews were conducted in Spanish by research interviewers trained in handling sensitive issues and had a mean duration of 57 min. The interview was created in English and translated to Spanish. In order to improve the reliability and validity of the final Spanish interview, it was back-translated to English by an independent translator and the original and back-translated documents were compared. The interview included assessment of several domains: demographics; migration history; social networks/support; HIV-related risk behavior; HIV testing history; history of alcohol/drug use; and health services utilization. Item response formats included nominal categories, raw numbers, yes/no, and 3- to 5-point Likert scales with explicit anchors, and included both open and closed-ended responses. The present study used questions (either as individual items or created scales) from each of these domains for description and/or use in the predictive model.
Dependent variable: late HIV testing
Late testing was defined as having at least one of: (1) an AIDS-defining illness within 1 year of first positive HIV test; (2) a date of AIDS diagnosis within 1 year of first positive HIV test; or (3) an initial CD4 cell count below 200 cells per microliter29 within 1 year of first positive HIV test.
Independent variables and scale creation
All independent variables used as potential correlates in the regression model (other than biological gender) were assessed using one of three time/recall periods. Marital status, education, and clinic recruitment site corresponded to the time of interview. Several variables were linked to the time of HIV diagnosis including age at HIV diagnosis, exposure category, stigma re: testing, testing site for first positive test, and items assessing barriers to testing earlier. All other variables in the model were assessed for the lifetime period prior to the first positive HIV test, using the following instructions: “Now I'm going to ask questions about the time before you were diagnosed with HIV. Please, try to think about the time before you found out you had HIV. That is, before ______ (year of HIV diagnosis).”
Prior to entry into the model, the distributional characteristics of all variables were inspected and adjustments were made to normalize distributions if necessary. The original formats of certain variables were recoded or transformed to this end. Some variables were also recoded in order to test a specific direction based on theory. The coding used to test all variables in the regression model is presented in the notes to Tables 1 and 2. Five of the variables in the model were created scales and are described below.
Table 1.
Descriptives for Potential Correlates of Late HIV Testing
| |
Prevalence sample n = 342 |
Model sample n = 275 |
||
|---|---|---|---|---|
| n (%) |
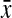 (SD; range) (SD; range)
|
n (%) |
 (SD; range) (SD; range)
|
|
| Clinic | ||||
| ISE Salud | 144 (42.1) | 122 (44.4) | ||
| IMSS | 198 (57.9) | 153 (55.6) | ||
| Biological gender | ||||
| Females | 110 (32.2) | 83 (30.2) | ||
| Males | 232 (67.8) | 192 (69.8) | ||
| Age at HIV diagnosisa | 34.44 (9.69; 9–69) | 34.12 (9.26; 18–69) | ||
| Marital status | ||||
| Married/cohabitating | 100 (29.2) | 79 (28.7) | ||
| Other | 242 (70.8) | 196 (71.3) | ||
| Years of educationa | 8.15 (3.93; 0–18) | 8.32 (3.99; 0–18) | ||
| Earned income | ||||
| Yes | 259 (75.7) | 208 (75.6) | ||
| No | 83 (24.3) | 67 (24.4) | ||
| HIV exposure category | ||||
| Heterosexual | 159 (46.5) | 116 (42.2) | ||
| Other | 183 (53.5) | 159 (57.8) | ||
| Frequency alcohol/drugs before sexa | 1.99 (0.72; 1–3) | 2.02 (0.70; 1–3) | ||
| Condom use | ||||
| Ever | 194 (58.1) | 167 (60.7) | ||
| Never | 140 (41.9) | 108 (39.3) | ||
| Type of regular healthcare provider | ||||
| Private physician in MX or CA | 77 (22.8) | 66 (24.0) | ||
| Otherb | 260 (77.2) | 209 (76.0) | ||
| Type of HIV tester | ||||
| Repeat | 154 (45.3) | 135 (49.1) | ||
| One-time | 186 (54.7) | 140 (50.9) | ||
| Peers with high-risk sexual practicesa | 8.01 (3.06; 2–15) | 7.98 (3.06; 2–15) | ||
| Peers with HIV infectionc | 2.89 (1.41; 1–8) | 3.0 (1.47; 1–8) | ||
| Exposure to preventive informationa | 12.26 (3.80; 5–24) | 12.60 (3.74; 5–24) | ||
| Site of first positive HIV test | ||||
| Private laboratory | 75 (22.1) | 63 (22.9) | ||
| Other | 265 (77.9) | 212 (77.1) | ||
| Stigma re: HIV-infected peopled | 15.16 (3.12; 2–18) | 15.28 (2.99; 3–18) | ||
| Stigma re: testinga | 0.85 (0.86; 0–2) | 0.88 (0.86; 0–2) | ||
| Delayed testing because … | ||||
| I didn't feel sick | ||||
| No | 94 (27.6) | 76 (27.6) | ||
| Yes | 246 (72.4) | 199 (72.4) | ||
| I wasn't told I should get tested | ||||
| No | 167 (49.1) | 141 (51.3) | ||
| Yes | 173 (50.9) | 134 (48.7) | ||
| I preferred not to know I had HIV | ||||
| No | 195 (57.5) | 154 (56.0) | ||
| Yes | 144 (42.5) | 121 (44.0) | ||
| I didn't know where to get tested | ||||
| No | 246 (72.4) | 203 (73.8) | ||
| Yes | 94 (27.6) | 72 (26.2) | ||
| I thought the test was expensive | ||||
| No | 221 (65.2) | 178 (64.7) | ||
| Yes | 118 (34.8) | 97 (35.3) | ||
Continuous variables.
Other includes clinics in Mexico and United States, general hospital in the United States, pharmacies, traditional healers, military hospitals/medical units, self-care/self-medicated, family members provided medications, knew someone in healthcare who provided medications, rehabilitation center, Red Cross, and also includes patients reporting no usual source of care.
The descriptives in the table for this variable correspond to the original data from the created scale. For analytical purposes, the variable was transformed using a natural logarithm to normalize a positively skewed distribution (for n = 342: mean = 0.96, SD = 0.44, range = 0–2.08; for n = 275: mean = 0.99, SD = 0.46, range = 0–2.08).
The descriptives in the table for this variable correspond to the original data from the created scale. For analytical purposes, this variable was recoded into tertiles to adjust for a negatively skewed distribution (for n = 342: mean = 2.11, SD = 0.85, range = 1–3; for n = 275: mean = 2.12, SD = 0.83, range = 1–3).
SD, standard deviation.
Table 2.
Full Model: Correlates of Late HIV Testing (n = 275)
| Determinant | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Clinica | 1.54 | 0.94–2.50 | 1.90 | 1.06–3.41 |
| Biological malesb | 1.51 | 0.89–2.58 | 1.53 | 0.70–3.35 |
| Age at HIV diagnosisc | 1.02 | 0.99–1.04 | 1.02 | 0.98–1.05 |
| Marital statusd | 1.33 | 0.79–2.26 | 1.64 | 0.85–3.15 |
| Years of educationc | 0.97 | 0.91–1.03 | 0.96 | 0.88–1.04 |
| Earned incomee | 1.17 | 0.67–2.04 | 1.73 | 0.88–3.41 |
| HIV exposure categoryf | 0.86 | 0.53–1.39 | 1.06 | 0.50–2.25 |
| Alcohol/drug use before sexc | 1.13 | 0.80–1.59 | 0.90 | 0.57–1.39 |
| Condom useg | 0.99 | 0.61–1.62 | 0.97 | 0.53–1.77 |
| Type of regular healthcare providerh | 1.32 | 0.76–2.30 | 1.34 | 0.70–2.54 |
| Type of HIV testeri | 0.91 | 0.57–1.47 | 0.88 | 0.50–1.52 |
| Peers with high-risk sexual practicesc | 1.06 | 0.98–1.14 | 1.14 | 1.02–1.27 |
| Peers with HIV infectionc | 0.96 | 0.57–1.62 | 0.73 | 0.37–1.42 |
| Exposure to preventive informationc | 0.99 | 0.93–1.06 | 0.98 | 0.91–1.06 |
| Site of first positive HIV testj | 1.25 | 0.71–2.20 | 1.62 | 0.80–3.26 |
| Stigma re: HIV-infected peoplek | 0.74 | 0.55–0.99 | 0.65 | 0.47–0.92 |
| Stigma re: testingc | 1.02 | 0.77–1.35 | 0.66 | 0.45–0.97 |
| Delayed testing because …l | ||||
| I didn't feel sick | 1.06 | 0.62–1.81 | 0.96 | 0.52–1.80 |
| I wasn't told I should get tested | 1.19 | 0.74–1.93 | 0.98 | 0.53–1.81 |
| I preferred not to know I had HIV | 2.13 | 1.31–3.47 | 2.78 | 1.46–5.31 |
| I didn't know where to get tested | 1.69 | 0.99–2.91 | 1.91 | 0.99–3.65 |
| I thought the test was expensive | 1.52 | 0.92–2.51 | 1.49 | 0.80–2.76 |
(1) IMSS (0) ISE SALUD; b(1) male and (0) female; cContinuous variables; d(1) Married/cohabitating and (0) other; e(1) No earned income and (0) yes; f(1) Heterosexual and (0) other; g(1) Never used and (0) ever used; h(1) private physician in Mexico or California and (0) other; i(1) one-time tester and (0) repeat tester; j(1) private laboratory and (0) other; kContinuous variable, recoded into tertiles to adjust for a negatively skewed distribution; l(1) yes and (0) no.
OR, odds ratio; CI, confidence interval.
Demographic variables included age, biological gender, marital status, number of years of education completed, and income. Health care utilization variables included: type of provider for main source of health care; HIV testing history; and testing site for first positive test.
Two scales were created to represent exposure to peers engaging in high-risk sexual behavior and having peers who had and/or had died from HIV/AIDS, respectively. Both of these scales were created from items using 5-point Likert scales (1 = none to 5 = all). The scale representing peers engaging in high-risk sexual behaviors (Cronbach α = 0.78) combined three items regarding the number of peers who: (1) had multiple partners; (2) had casual sex; and (3) had sex under the influence of alcohol/drugs. The HIV-infected peers scale (α = 0.72) contained two items assessing the number of peers who: (1) had HIV infection and (2) had died from AIDS.
A frequency of exposure to HIV preventive information prevention scale (α = 0.67) was created using six 4-point items (1 = never to 4 = often) assessing how often participants heard HIV preventive messages from: radio; television; a doctor or health care professional; friends; partner(s); and other places. Two stigma scales were created. A scale measuring the stigma regarding HIV-infected people (α = 0.70) was created using six 3-point Likert scale questions (1 = don't agree at all to 3 = completely agree) adapted from a HIV stigma scale developed by Berger et al.30 Examples of the items included in the created scale are: “Most people with HIV were rejected when others found out”; “Most people were uncomfortable around someone with HIV”; and “People with HIV lost their jobs when their employers found out.” A second stigma scale (α = 0.70) assessing stigma regarding testing was created from two yes/no items: (1) “I felt ashamed to seek HIV testing” and (2) “I was afraid that others could find out I had HIV infection.”
Risk practices for HIV included: HIV exposure category; practicing sex under the influence of alcohol/drugs; and condom use history before HIV diagnosis. The sex under the influence question used a 3-point Likert scale (1 = never to 3 = always) and was worded, “Before you were diagnosed with HIV infection, how often did you drink alcohol or use drugs before having sex?” The 4-items included in the condom scale used 3-point Likert response scales (1 = never to 3 = always). The vaginal and receptive anal sex questions used the wording, “Before your diagnosis of HIV, how often did you and your partner use a condom, when ______?” The oral and insertive anal sex questions were phrased, “Before (year: ______), how often did you use a condom when ______?” For analysis the condom use scale was dichotomized to correct a positive skew. Perceived barriers to testing included yes/no variables assessing: (1) not feeling sick; (2) not being told that should get tested; (3) preferring not to know one had HIV; (4) not knowing where to get tested; and thinking the HIV test was expensive.
Statistical analyses
Descriptive statistics for late HIV testing and potential correlates were computed. Logistic regression was used to identify potential correlates of late diagnosis. Regression diagnostic procedures were conducted and yielded no evidence of collinearity. Bivariate analyses were used to compare characteristics of the surveyed and not-surveyed subsamples to evaluate the representativeness of the sample. All statistical analyses were performed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL).
Results
Comparability of the sample
The 362 interviewed participants were compared to the remaining 308 HIV-positive patients who were not interviewed on socio-demographic characteristics and other key variables. Three significant differences were found. A significantly higher proportion of women were in the surveyed group (31.5% versus 18.8%), χ2(1, N = 670) = 13.98, p < 0.001. Significantly fewer participants in the surveyed group were late testers (43.3% versus 51.6%), χ2(1, N = 670) = 4.31, p < 0.05 and the surveyed group had significantly fewer participants recruited from IMSS (55.2% versus 69.2%), χ2(1, N = 670) = 13.61, p < 0.001. No differences were found by mean age at HIV diagnosis, educational level, marital status, HIV risk category, or mean number of years since HIV diagnosis.
Prevalence of late HIV testing and clinical characteristics
The prevalence of late HIV diagnosis for the 342 participants for whom late testing could be determined was 43.2%. The mean number of years since first HIV diagnosis was 3.9 (standard deviation [SD] = 3.6; range, 1–19). Over half (59.5%) of the patients were asymptomatic when first diagnosed with HIV, 45.2% had an initial CD4 count less than 200 cells per microliter, while 12.9% had an initial CD4 count greater than or equal to 500 cells per microliter. The majority (92.4%) acquired HIV infection via sexual transmission and 89.5% were receiving HAART at the time of the interview.
Among the 148 diagnosed late, 87.1% had an initial CD4 count less than 200 cells per microliter, 0.0% had an initial CD4 count greater than or equal to 500 cells per microliter, and 78.9% had AIDS within 6 months of their first HIV-positive test. Almost half (51.4%) had an AIDS-defining illness diagnosed concurrently with HIV, most commonly Mycobacterium tuberculosis (38.6%) wasting syndrome (21.6%), or Candida of the bronchi, lungs, trachea, and/or esophagus (14.9%). Of the 194 who did not test late, 13.4% had an initial CD4 count less than 200 cells per microliter and 22.7% had an initial CD4 count greater than or equal to 500 cells per microliter.
Correlates of late HIV testing
With all variables included in the model, the analyzable n size for predicting late HIV testing was 275. Descriptive information for the independent variables included in the predictive model is presented in Table 1. The overall model significantly explained an estimated 19% of the variance in late testing (based on Nagelkerke R2), χ2(22, N = 275) = 41.61, p < 0.01 and met Hosmer and Lemeshow goodness of fit standards, χ2(8, N = 275) = 2.74, p = 0.95.
Multivariate logistic regression results (Table 2) revealed five significant correlates of late HIV testing. The first was a reason for delaying testing: “I preferred not to know I had HIV” (AOR 2.78 [95% confidence interval {CI} 1.46–5.31] p = 0.002). The other significant correlates were: clinic of recruitment (AOR 1.90 [95% CI 1.06–3.41] p = 0.031); exposure to peers engaging in high-risk sexual behavior (AOR 1.14 [95% CI 1.02–1.27] p = 0.017); stigma regarding HIV-infected individuals (AOR 0.65 [95% CI 0.47–0.92] p = 0.014); and stigma regarding HIV testing (AOR 0.66 [95% CI 0.45–0.97] p = 0.035). “I didn't know where to get tested” (AOR 1.91 [95% CI 0.99–3.65], p = 0.052) was near significant.
Discussion
While antiretroviral therapy has been successful in delaying progression to AIDS, late HIV testing remains a contributing factor to the incidence of AIDS. Studies have examined the prevalence and correlates of late testing in several countries including Australia,2 China,7 France,15 Italy,4 Spain,5 the United Kingdom and Ireland,18 the United States,13,20 and Venezuela.16 To our knowledge this study is the first to examine the prevalence and correlates of late HIV testing in Mexico and the first to use an ecological model of behavior to examine potential correlates of late HIV testing.
The results show that 43.2% of our participants tested late. This rate falls within the range (19.2–51%) of late HIV testing and diagnosis prevalence found by other studies in the United States, the United Kingdom and Ireland, France, Spain, Italy, China, Canada, and Venezuela.5–7,13,15,16,18,19,31 The difference in the rates of late HIV testing and diagnosis across studies can be partially explained by variations in the definition of late HIV diagnosis.
Studies in California have found that Latino populations predominantly from Mexican origin delay HIV testing and entry into medical care more frequently than other ethnic groups,22,31 as indicated by the disproportionate percentage of AIDS cases and increased AIDS incidence among this population.32,33 The majority of our participants were found to be immigrants to Tijuana from other Mexican regions. The 43.2% prevalence rate of late HIV testing from this study also falls within the range of 23–49% found among Latinos in the United States.20,32
The strongest correlate of late HIV diagnosis was delayed testing due to “preferring not to know I had HIV.” This result is consistent with findings from a study conducted in France, in which “prefer not to know” was the only reason for not previously testing stated significantly more frequently by late testers than other AIDS patients.15 Future studies need to explore this concept in depth, including examination of the reasons for preferring not to know. Better understanding of the mechanisms which drive people to delay testing because they prefer not to know if they are HIV positive may inform interventions to help increase early testing and diagnosis in these individuals.
Participants recruited from IMSS had nearly twice the odds of testing late. This finding suggests that participants with employer-based health insurance and seeking care at this facility designated to serve this population were more likely to be late testers. However, it does not necessarily mean that participants had their first positive HIV test there, but rather merely that they were receiving care at IMSS as the time of recruitment for this study. For those patients who did test positive for the first time at this clinic, this result is consistent with findings from other studies related to late HIV testing. One study conducted in the United States found that having private health insurance at the time of diagnosis was a strong predictor of late testing.13 Another study among sexually experienced young adults found that those who had private health insurance were less likely to self-report HIV testing in the past 12 months.33 Similarly, a study among emergency department patients found that those who had private health insurance were less likely to have been tested previously for HIV.34 Findings related to health insurance status at the time of HIV diagnosis should be further investigated.
Exposure to peers engaging in high-risk sexual behaviors was a significant correlate of testing late, which is consistent with the BEM and may represent a response to a subculture of peer pressure23 and exposure to social models for which risk behaviors and HIV infection is the norm. Future research should explore these mechanisms and how such influences might be reversed.
Surprisingly, both stigma regarding HIV-infected people and stigma regarding HIV testing emerged as significant protective factors for late testing. These relationships were confirmed by further bivariate exploration of our stigma scales with HIV testing. These findings contradict our hypotheses related to stigma based on the BEM as well as extensive literature indicating that stigma is associated with delayed testing or not testing at all.35–40 Our results suggest that the higher levels of stigma found in early testers compared to late testers, may be an indication of stigmatizing attitudes resulting from the testing experience, however, our findings related to stigma should be investigated in future research.
Although near significant, individuals who did not know where to get tested for HIV had nearly twice the odds of testing late for HIV. In a Chinese study examining correlates of ever testing for HIV in MSM, not knowing the location of test sites was the second most common reason for not testing.41
Limitations of this study include the cross-sectional nature of the interview data and reliance on self-reported questionnaire measures. Other limitations included the inability to interview almost half (46%) of those participants for whom charts were abstracted due to missed clinic visits, being too sick to participate, not being referred by medical staff, having cognitive impairments, being unable to verbally communicate, being in prison, refusing to participate (the small percent of refusals does not appear to have had any adverse effects on our conclusions), or death. Thus, the results may not be generalizable to all HIV positive people ever treated for HIV/AIDS in the two clinics or to individuals living with HIV/AIDS in Tijuana.
Our findings may inform future research as well as the design of interventions to increase timely HIV testing and diagnosis. Such interventions should promote the benefits of early diagnosis and also aim at changing social views about the normalcy of HIV testing as well as about the treatability of HIV infection. If HIV testing is made readily available both in terms of cost and location of testing sites, the opportunities for HIV-infected persons to know their serostatus may increase. Incorporating HIV testing into routine medical care is consistent with CDC's initiative and could be effective in increasing HIV testing in social networks where risk is not the norm, while continuing to promote HIV testing among higher risk groups. Furthermore, including routine HIV testing as part of regular medical care may contribute to the reduction of HIV transmission in the community at large.
Acknowledgments
This research was supported by grants awarded to Melbourne F. Hovell, Ph.D., M.P.H from the California HIV/AIDS Research Program, University of California (#IS99-SDSUF-206 and #IS02-CBECH-711). The study sponsors had no role in the study design, data collection, data analyses, interpretation of the data, writing of the report, or the decision to submit the article for publication. All researchers are independent from the study funder.
The authors thank the medical staff at the Instituto Mexicano del Seguro Social, Hospital General Regional No.20 and the Secretaria de Salud, Hospital General de Tijuana. Special gratitude is extended to the interviewers and participants for their valuable contributions.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sanders GD. Bayoumi AM. Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–585. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 2.McDonald AM. Li Y. Dore GJ. Ree H. Kaldor JM. Late HIV presentation among AIDS cases in Australia, 1992–2001. Aust N Z J Public Health. 2003;27:608–613. doi: 10.1111/j.1467-842x.2003.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 3.Longo B. Pezzotti P. Boros S. Urciuoli R. Rezza G. Increasing proportion of late testers among AIDS cases in Italy, 1996–2002. AIDS Care. 2005;17:834–841. doi: 10.1080/09540120500038397. [DOI] [PubMed] [Google Scholar]
- 4.Girardi E. Sampaolesi A. Gentile M. Nurra G. Ippolito G. Increasing proportion of late diagnosis of HIV infection among patients with AIDS in Italy following introduction of combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;25:71–76. doi: 10.1097/00042560-200009010-00010. [DOI] [PubMed] [Google Scholar]
- 5.Castilla J. Sobrino P. de la Fuente L. Noguer I. Guerra L. Parras F. Late diagnosis of HIV infection in the era of highly active antiretroviral therapy: Consequences for AIDS incidence. AIDS. 2002;16:1945–1951. doi: 10.1097/00002030-200209270-00012. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm. [Dec 14;2009 ];MMWR Morb Mortal Wkly Rep. 2006 55:1–17. [PubMed] [Google Scholar]
- 7.Wong KH. Lu S. Lee SS. Low K. Wan WY. Temporal trend and factors associated with late HIV diagnosis in Hong Kong, a low HIV prevalence locality. AIDS Patient Care STDs. 2003;17:461–469. doi: 10.1089/108729103322395492. [DOI] [PubMed] [Google Scholar]
- 8.Health Profile: Mexico HIV/AIDS. US Agency for International Development. Washington, D.C.: 2005. [Dec 14;2009 ]. [Google Scholar]
- 9.Magis-Rodríguez C. Bravo-García E. Uribe-Zúñiga P. México DF: Centro Nacional de la Prevención y Control del SIDA; [Dec 14;2009 ]. Dos décadas de la epidemia del SIDA en México. [Google Scholar]
- 10.SS/DGE. Registro Nacional de Casos de SIDA. Datos al 15 de noviembre del 2007 y 14 de noviembre de 2008. Casos nuevos y acumulados de sida por año de diagnóstico, según entidad federativa. www.censida.salud.gob.mx/descargas/2008/casosentidadnov.pdf. [Dec 14;2009 ]. www.censida.salud.gob.mx/descargas/2008/casosentidadnov.pdf
- 11.Panorama Epidemiológico del VIH/SIDA e ITS en México. México DF.: Centro Nacional de la Prevención y Control del SIDA; 2006. [Dec 14;2009 ]. [Google Scholar]
- 12.II Conteo de Población y Vivienda. México DF: 2005. [Dec 14;2009 ]. Instituto Nacional de Estadística, Geografía e Informática (INEGI) [Google Scholar]
- 13.Schwarcz S. Hsu L. Dilley JW. Loeb L. Nelson K. Boyd S. Late diagnosis of HIV infection. J Acquir Immune Defic Syndr. 2006;43:491–494. doi: 10.1097/01.qai.0000243114.37035.de. [DOI] [PubMed] [Google Scholar]
- 14.Mayben JK. Kramer JR. Kallen MA. Franzini L. Lairson DR. Ciordano TP. Predictors of delayed HIV diagnosis in a recently diagnosed cohort. AIDS Patient Care STDs. 2007;21:195–204. doi: 10.1089/apc.2006.0097. [DOI] [PubMed] [Google Scholar]
- 15.Couturier E. Schwoebel V. Michon C, et al. Determinants of delayed diagnosis of HIV infection in France, 1993–1995. AIDS. 1998;12:795–800. doi: 10.1097/00002030-199807000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Bonjour MA. Montagne M. Zambrano M, et al. Determinants of late disease-stage presentation at diagnosis of HIV infection in Venezuela: A case-case comparison. www.aidsrestherapy.com/content/5/1/6. [Jun 23;2009 ];AIDS Res Ther. 2008 5:6. doi: 10.1186/1742-6405-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mugavero MJ. Castellano C. Edelman D. Hicks C. Late diagnosis of HIV infection: The role of age and sex. Am J Med. 2007;120:370–373. doi: 10.1016/j.amjmed.2006.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan AK. Curtis H. Sabin CA. Johnson MA. Newly diagnosed HIV infections: review in UK and Ireland. BMJ. 2005;330:1301–1302. doi: 10.1136/bmj.38398.590602.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plitt SS. Mihalicz D. Singh AE. Jayaraman G. Houston S. Lee BE. Time to testing and accessing care among a population of newly diagnosed patients with HIV with a high promotion of Canadian aboriginals, 1998–2003. AIDS Patient Care STDs. 2009;23:93–99. doi: 10.1089/apc.2007.0238. [DOI] [PubMed] [Google Scholar]
- 20.CDC. Late versus early testing of HIV—16 sites, United States, 2000–2003. www.cdc.gov/mmwr/preview/mmwrhtml/mm5225a2.htm. [Dec 14;2009 ];MMWR Morb Mortal Wkly Rep. 2003 52:581–586. [PubMed] [Google Scholar]
- 21.Vermund SH. Wilson CM. Barriers to HIV testing—Where next? Lancet. 2002;360:1186–1187. doi: 10.1016/S0140-6736(02)11291-8. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Quintero C. Shtarkshall R. Neumark YD. Barriers to HIV-testing among Hispanics in the United States: Analysis of the National Health Interview survey, 2000. AIDS Patient Care STDs. 2005;19:672–683. doi: 10.1089/apc.2005.19.672. [DOI] [PubMed] [Google Scholar]
- 23.Hovell MF. Wahlgren DR. Gehrman CA. The Behavioral Ecological Model: Integrating public health and behavioral science. In: DiClemente RJ, editor. Emerging Theories in Health Promotion Practice and Research: Strategies for Improving Public Health. San Francisco, CA; Jossey-Bass: 2002. pp. 347–385. [Google Scholar]
- 24.Hovell MF. Wahlgren DR. Adams MA. The logical and empirical basis for the Behavioral Ecological Model. In: DiClemente RJ, editor; Crosby RA, editor; Kegler M, editor. Emerging Theories in Health Promotion Practice and Research: Strategies for Improving Public Health. 2nd. San Francisco, CA: Jossey-Bass; 2009. pp. 415–449. [Google Scholar]
- 25.Hovell MF. Hillman ER. Blumberg E, et al. A behavioral-ecological model of adolescent sexual development: A template for AIDS prevention. J Sex Res. 1994;31:267–281. [Google Scholar]
- 26.Adams MA. Hovell MF. Irvin V, et al. Promoting stair use by modeling: An experimental application of the Behavioral Ecological Model. Am J Health Promot. 2006;21:101–109. doi: 10.4278/0890-1171-21.2.101. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Donate AP. Hovell MF. Hofstetter CR. Gonzalez-Perez G. Adams MA. Kotay A. Correlates of home smoking bans among Mexican-Americans. Am J Health Promot. 2007;21:229–236. doi: 10.4278/0890-1171-21.4.229. [DOI] [PubMed] [Google Scholar]
- 28.Blumberg EJ. Liles S. Kelley NJ, et al. Predictors of weapon-carrying in youth attending drop-in centers. Am J Health Behav. 2009;33:745–758. doi: 10.5993/ajhb.33.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC. The 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. www.cdc.gov/MMWR/preview/mmwrhtml/00018871.htm. [Dec 14;2009 ];MMWR Morb Mortal Wkly Rep. 1992 41:RR-17. [PubMed] [Google Scholar]
- 30.Berger BE. Ferrans CE. Lashley FR. Measuring stigma in people with HIV: Psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24:518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 31.Neal JJ. Fleming PL. Frequency and predictors of late HIV diagnosis in the United States, 1994 through 1999. Proceedings of the 9th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. Feb 24–28, 2002. [Dec 14;2009 ]. [Google Scholar]
- 32.Samet JH. Freedberg KA. Savetsky JB. Sullivan LM. Stein MD. Understanding delay to medical care for HIV infection: The long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TQ. Ford CA. Kaufman JS, et al. HIV testing among young adults in the United States: Associations with financial resources and geography. Am J Public Health. 2006;96:1031–1034. doi: 10.2105/AJPH.2005.063248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merchant RC. Catanzaro BM. Seage GR., 3rd Demographic variations in HIV testing history among emergency department patients: Implications for HIV screening in US emergency departments. J Med Screen. 2009;16:60–66. doi: 10.1258/jms.2009.008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solorio MR. Currier J. Cunningham W. HIV health care services for Mexican migrants. J Acquir Immune Defic Syndr. 2004;37(Suppl 4):S240–S251. doi: 10.1097/01.qai.0000141251.16099.74. [DOI] [PubMed] [Google Scholar]
- 36.Brimlow DL. Cook JS. Seaton R. Stigma and HIV/AIDS: A review of the literature. Rockville, MD: US Department of Health and Human Services; 2003. [Dec 14;2009 ]. [Google Scholar]
- 37.Ritieni A. Moskowitz J. Tholandi M. HIV/AIDS misconceptions among Latinos: Findings from a population-based survey of California adults. Health Educ Behav. 2008;35:245–259. doi: 10.1177/1090198106288795. [DOI] [PubMed] [Google Scholar]
- 38.CDC. HIV-related knowledge and stigma United States, 2000. www.cdc.gov/mmwr/preview/mmwrhtml/mm4947a2.htm. [Dec 14;2009 ];MMWR Morb Mortal Wkly Rep. 2000 49:1062–1064. [PubMed] [Google Scholar]
- 39.Kalichman SD. Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counseling and testing in a black township in Cape Town, South Africa. Sex Transm Infect. 2003;79:442–447. doi: 10.1136/sti.79.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiser SD. Heisler M. Leiter K, et al. Routine HIV testing in Botswana: A population-based study on attitudes, practices, and human rights concerns. www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0030261. [Dec 14;2009 ];PLoS Med. 2006 3:e261. doi: 10.1371/journal.pmed.0030261. Erratum in: PLoS Med 2006;3:e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi KH. Hui L. Guo Y. Han L. Mandel J. Lack of HIV testing and awareness of HIV infection among men who have sex with men, Beijing, China. AIDS Educ Prev. 2006;18:33–43. doi: 10.1521/aeap.2006.18.1.33. [DOI] [PubMed] [Google Scholar]


