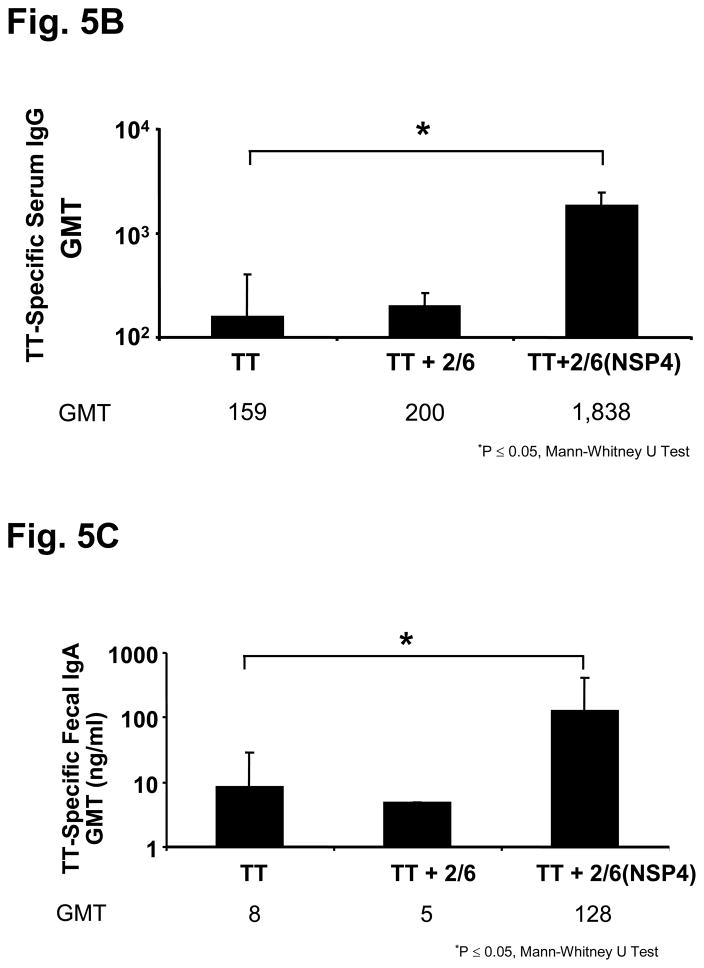
Figure 5.
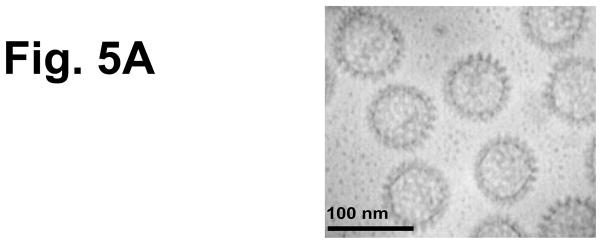
Characterization of 2/6-VLP containing NSP4 and TT-specific serum and fecal antibody response profiles of mice intranasally co-administered TT with rotavirus 2/6 VLP containing NSP4. To test another form of NSP4 obtained from a system other than the baculovirus-expressed and affinity-purified SA11 NSP4-containing 2/6 VLPs were used as immunogens. (a) Negative stain (1% uranyl acetate, pH4.5) electron micrographs of NSP4-2/6 VLP produced in insect Sf9 cells. Magnification bar equals 100nm. Animals (n=5) were given TT alone or co-administered with either 2/6 VLP alone or 2/6 VLP containing NSP4 three times two weeks apart. Samples were collected 14 days post final inoculation and assayed by ELISA TT-specific serum IgG (b) and fecal IgA (c) were evaluated for enhanced responses compared to the antigen alone group.