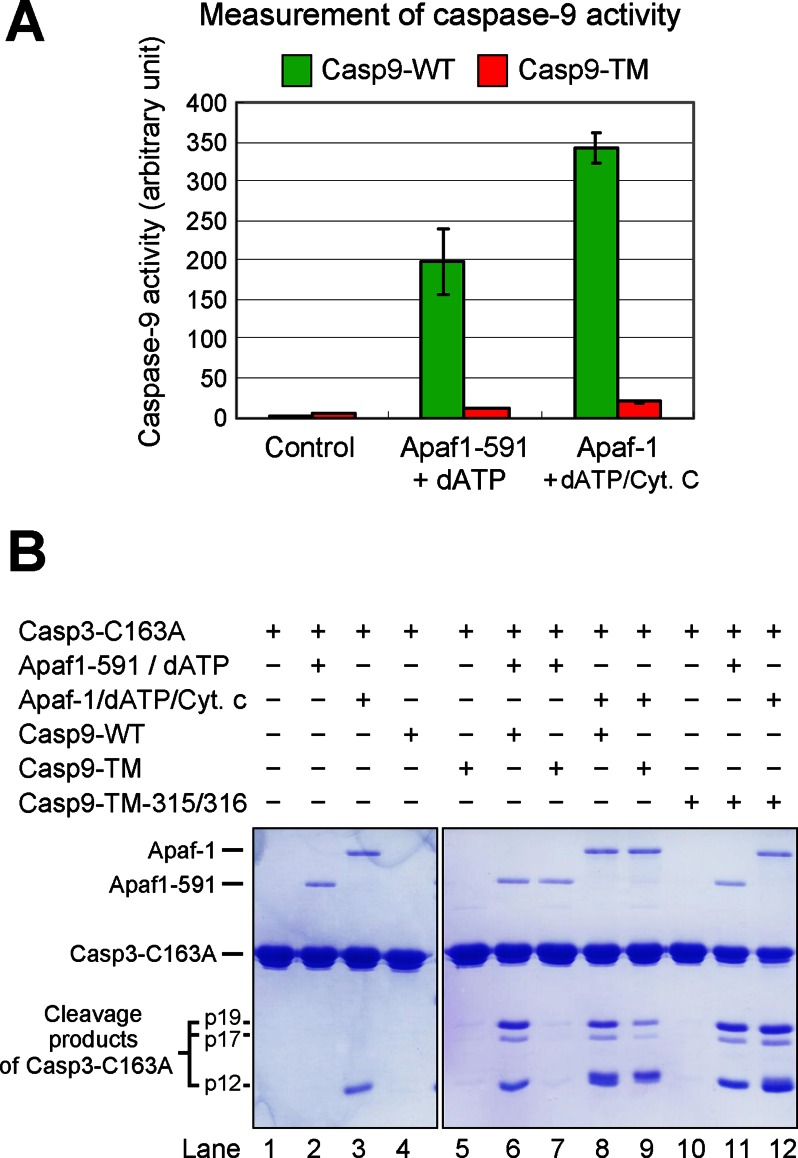
FIGURE 2.
WT caspase-9, but not Casp9-TM, is appropriately activated by the apoptosome. A, direct measurement of caspase-9 activity using the caspase-9-specific peptide substrate Ac-LEHD-AFC. The protease activity of WT caspase-9, but not uncleavable Casp9-TM, was markedly stimulated by the apoptosome formed by either Apaf-1(1–591) (Apaf1-591) or full-length Apaf-1. B, direct measurement of caspase-9 activity using caspase-3(C163A) as the substrate. Shown is an SDS-polyacrylamide gel stained with Coomassie Blue. Although the substrate protein was also cleaved by Casp9-TM in the presence of Apaf-1/cytochrome c (Cyt. c)/dATP (lane 9), the extent of cleavage was much smaller compared with WT caspase-9 (lane 8). Because the position of cytochrome c on the SDS-polyacrylamide gel nearly coincides with that of the p12 cleavage fragment, the majority of the lower band in lane 9 was contributed by cytochrome c.