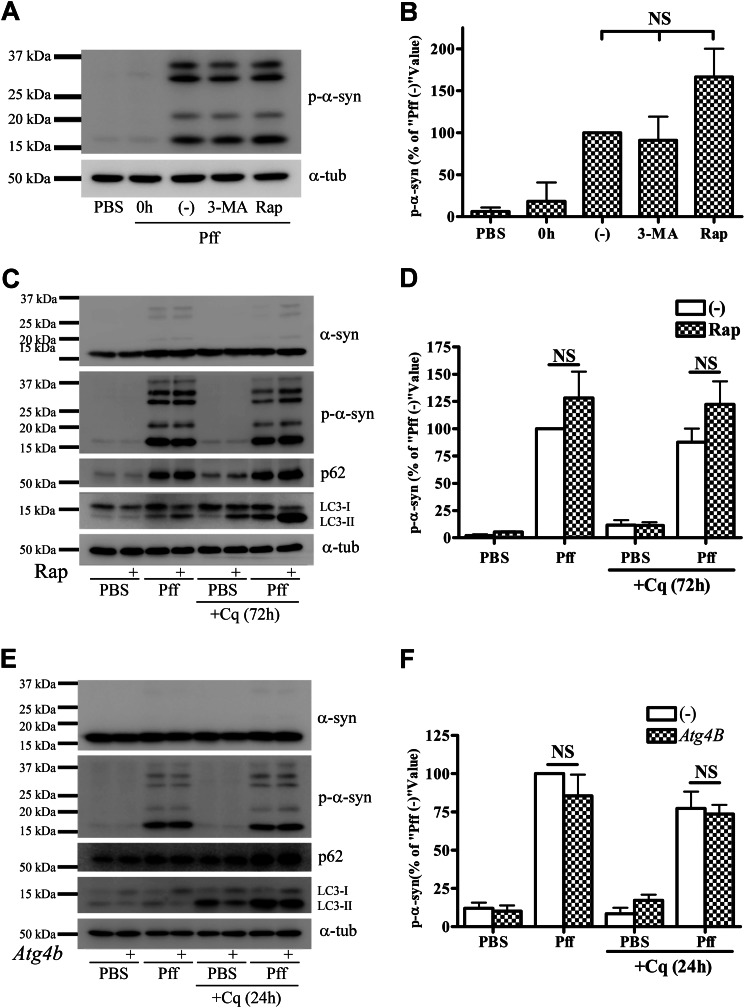
FIGURE 2.
Autophagy does not degrade α-syn aggregates. A and B, after 4 h of Pff transduction, HEK293 α-syn cells were treated with the autophagy inhibitor 3-MA (10 mm) or the autophagy activator Rap (0.2 μm) in starvation media for 24 h. Cells were subsequently extracted with SDS for IB. A, representative IB of p-α-syn and α-tubulin (α-tub), and B, quantification of IBs (n = 3). “0h” refers to cells that were harvested prior to drug treatment, and “(−)” refers to vehicle-treated cells. Minimal α-syn pathology was present when drug treatment was initiated, as shown in the 0h lane. Neither 3-MA nor rapamycin caused a significant change in p-α-syn levels. C and D, extended rapamycin treatment in a nutrient-rich condition does not induce clearance of aggregates. 4 h after Pff transduction, HEK293 α-syn cells were maintained in nutrient-rich media for 20 h, followed by rapamycin treatment for 72 h in the absence or presence of 15 μm Cq in nutrient-rich conditions. C, IB of p-α-syn, p62, LC3, and the loading control, α-tubulin. D, quantification of p-α-syn levels in IBs from three independent experiments, with data normalized to the mean p-α-syn level from vehicle-treated Pff-td cells. Significant activation of autophagy by rapamycin was evident because LC3-II accumulated in Cq and rapamycin co-treated cells, but this activation did not reduce aggregated α-syn levels. E and F, genetic inhibition of autophagy does not cause accumulation of p-α-syn aggregates. HEK293 α-syn-stable cells were transfected with the dominant negative autophagy inhibitor, mutant ATG4BC74A-strawberry, or with empty vector (−). Cells were transduced with Pff for 24 h, treated with 30 μm Cq for an additional 24 h, and then harvested 72 h after transfection. E, IB of p-α-syn, p62, LC3, and the loading control, α-tubulin. F, quantification of p-α-syn levels from three independent experiments, with data normalized to the mean p-α-syn level from empty vector-transfected Pff-td cells. ATG4B mutant (ATG4BC74A) prevents lipidation of LC3 to LC3-II, thereby inhibiting autophagosome closure and autophagy (38) resulting in a significant decrease in total and LC3-I-normalized LC3-II levels in ATG4B-transfected cells (2.34 ± 0.30 versus 0.63 ± 0.05 in Pff-td mock and ATG4B-transfected cells, respectively, p < 0.01) without changing aggregated p-α-syn levels. Error bars, ± S.E. One-way ANOVA with Tukey's post-hoc analysis (B) or Student's paired t test (D and F) was used to test for statistically significant differences. NS indicates p > 0.05.