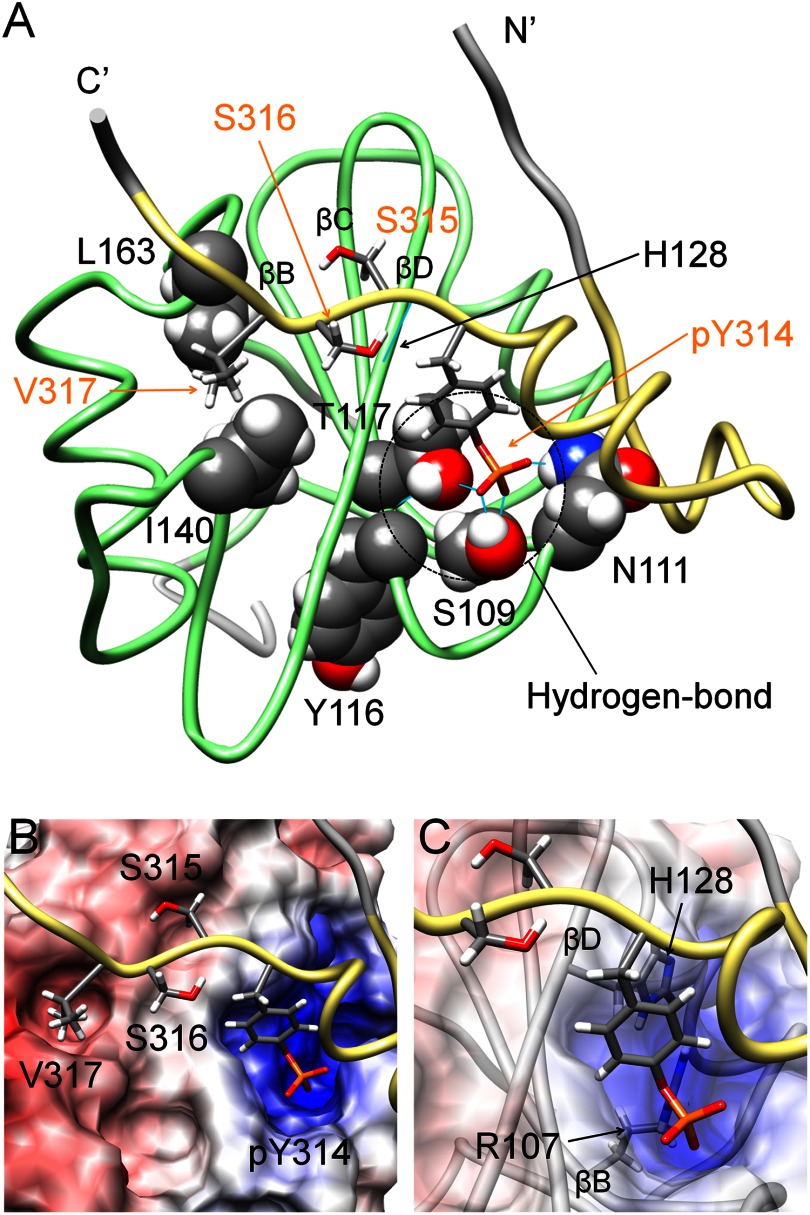
FIGURE 4.
Conventional interaction mode is observed in the structure of the complex of Csk-SH2 with Cbp5. A, tube representation of the complex of Csk-SH2 with Cbp5. Amino acid residues involved in the interaction are represented using a stick model for Cbp5 and a sphere model for Csk-SH2. Hydrogen bonds are shown as cyan lines and those around Tyr(P)-314 are highlighted with dotted circles. Amino acid residues of Cbp5 and Csk-SH2 are labeled in orange and black, respectively. B, binding pockets on the surface of Csk-SH2 with residues around Tyr(P)-314 and Val-317 of Cbp5. The electrostatic potential was calculated using Delphi and mapped on the surface of the structure of Csk-SH2. Positive and negative charges are indicated in blue and red, respectively. C, Arg-107 and His-128 of Csk-SH2, which generates the positive charge around Tyr(P)-314, interact with the phosphate group of Tyr(P)-314 in the conventional manner.