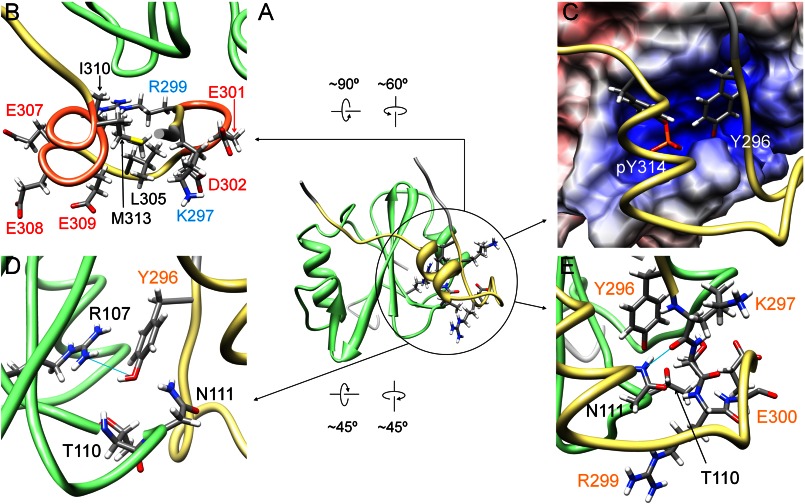
FIGURE 5.
Another interaction region found in the structure of the complex of Csk-SH2 with Cbp5. A, interaction region between Csk-SH2 and Cbp5 is circled in black in the ribbon diagram of the complex of Cbp5 with Csk-SH2. Amino acid residues involved in the interaction are represented using a stick model. B, Cbp5 exhibits an amphipathic character, with hydrophilic residues exposed to the solvent and hydrophobic residues and aliphatic chains oriented inward. Positively and negatively charged residues are labeled in cyan and red, respectively, and hydrophobic residues are labeled in black. C, Tyr-296 and Tyr(P)-314 of Cbp5 (in khaki) are accommodated in the binding pocket of Csk-SH2. The two residues are represented using a stick model. An α-helix and a turn of Cbp formed on Csk-SH2 bring the two Tyr residues close to each other. D, residues of Csk-SH2 interacting electrostatically with the hydroxyl group of Tyr-296. Hydrogen bonds are shown by cyan lines. Amino acid residues of Cbp5 and Csk-SH2 are labeled in orange and black, respectively. E, residues additionally found as contributors to the second interaction site. Amino acid residues of Cbp5 and Csk-SH2 are labeled in orange and black, respectively. Hydrogen bonds are shown as cyan lines. Csk-SH2 is shown in green and Cbp5 in khaki.