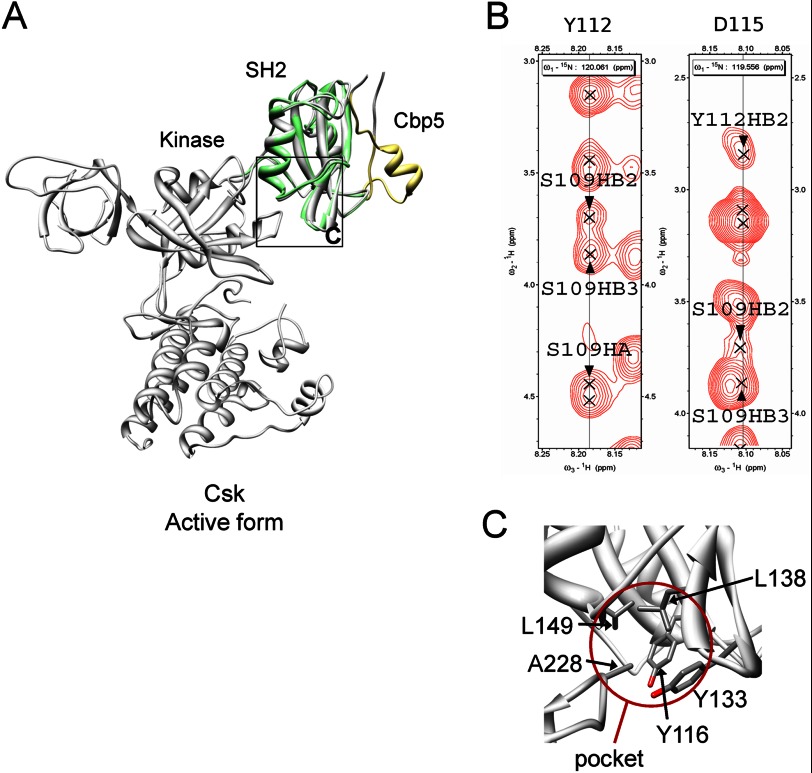
FIGURE 9.
Connection between Cbp5 and the kinase domain through Csk-SH2. A, relative configuration between Cbp5 and the kinase domain revealed by overlaying of the solution structure of Csk-SH2 in complex with Cbp5 and the crystal structure of intact Csk (PDB code 1K9A). Csk-SH2 and Cbp5 in the complex are colored in green and khaki, respectively, and intact Csk is colored in gray. The region corresponding to C is shown with a black square. B, intramolecular NOEs observed among Ser-109, Tyr-112, and Asp-115 supporting the presence of the hydrogen bonds between them. The corresponding regions are shown from a 15N-edited NOESY spectrum of 15N-labeled Csk-SH2 in complex with unlabeled Cbp5. C, insertion of Ala-228 into the hydrophobic pocket formed by Tyr-116, Tyr-133, Leu-138, and Leu-149, as observed in the crystal structure of Csk. These residues are shown with a stick model. The pocket is highlighted with a brown circle.