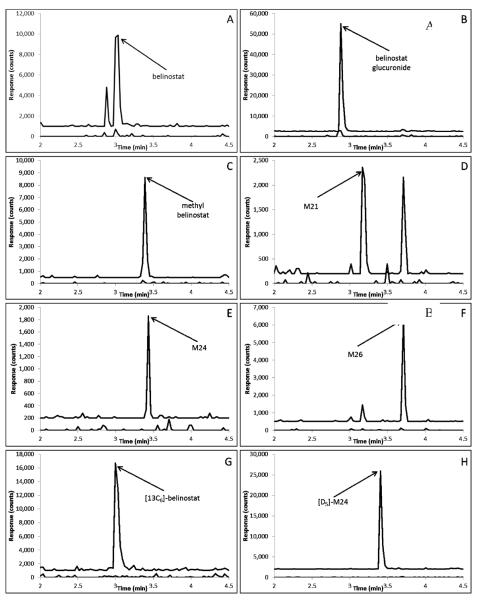
Fig. 2.
Representative chromatograms of: A) belinostat (m/z 319.1>93.0; 2.94 min) added to control plasma at the LLQ concentration of 30 ng/mL (top trace with an offset of 1000 counts) and control human plasma (bottom trace); B) belinostat glucuronide (495.3>319.1; 2.82 min) added to control plasma at the LLQ concentration of 30 ng/mL (top trace with an offset of 2500 counts) and control human plasma (bottom trace); C) methyl belinostat (m/z), 333.1>93.0; 3.30 min)) added to control plasma at the LLQ concentration of 30 ng/mL (top trace with an offset of 500 counts) and control human plasma (bottom trace); D) M21 (m/z 301.1>92.0; 3.10) added to control plasma at the LLQ concentration of 30 ng/mL (top trace with an offset of 200 counts) and control human plasma (bottom trace); E) M24 (m/z 273.1>92.0; 3.38 min) added to control plasma at the LLQ concentration of 30 ng/mL (top trace with an offset of 200 counts) and control human plasma (bottom trace); F) M26 (302.1>92.2; 3.62min) added to control plasma at the LLQ concentration of 30 ng/mL (top trace with an offset of 500 counts) and control human plasma (bottom trace); G) [13C6]-belinostat internal standard (m/z 325.1>99.0; 2.94 min) added to control plasma at a concentration of 200 ng/mL (top trace with an offset of 1000 counts) and control human plasma (bottom trace); H) [D5]-M24 internal standard (m/z 281.2>97.3; 3.32 min) added to control plasma at a concentration of 40 ng/mL (top trace with an offset of 2000 counts) and Scontrol human plasma (bottom trace).