Table 2.
Syntheses of (E)-δ-Silyl-anti-homoallylic Alcohols 6a
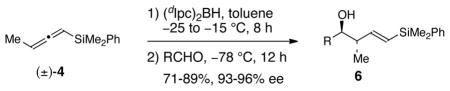
| ||||
|---|---|---|---|---|
| entry | RCHO | product | yieldb | % eec |
| 1 | PhCHO | 6a | 85% | 95 |
| 2d | PhCHO | ent-6a | 82% | 94 |
| 3 | PhCH=CHCHO | 6b | 78% | 95 |
| 4 | Ph(CH2)2CHO | 6c | 89% | 93 |
| 5 | CyCHO | 6d | 71% | 94 |
| 6 | TBSO(CH2)2CHO | 6e | 72% | 95 |
| 7 | BnOCH2CHO | 6f | 75% | 96 |
Reactions were performed by treating (±)-4 with (dIpc)2BH (1 equiv) in toluene at −25 °C and warming to −15 °C over 8 h followed by the addition of RCHO (0.45 equiv) at −78 °C. The mixture was then allowed to stir at −78 °C for 12 h. The reactions were subjected to a standard workup (NaHCO3, H2O2) at 0 °C prior to product isolation.
Based on the amount of the aldehydes used in the crotylboration reaction.
Determined by Mosher ester analysis.9
(lIpc)2BH was used for the hydroboration reaction.
