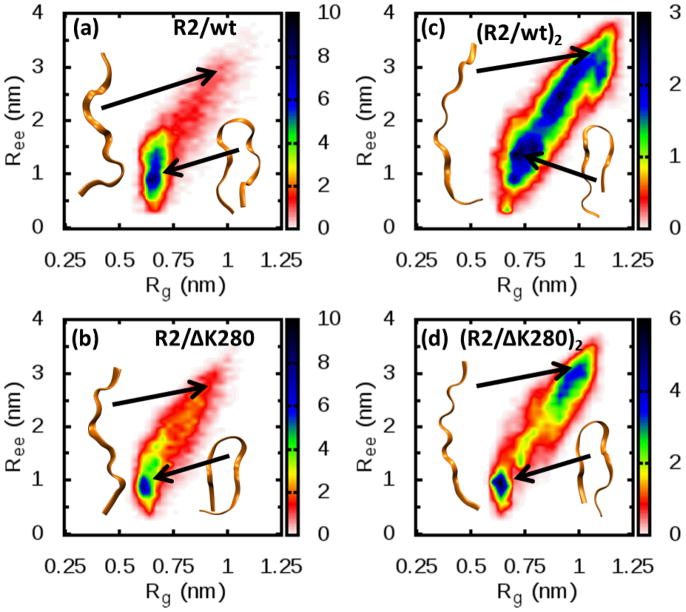
Fig 6.
Normalized probability distribution of the conformation for one molecule of R2/wt or R2/ΔK280 in solution. The data pertain to the peptides in their natural charged state, namely z/n=+2/1 for R2/wt and z/n=+1/1 for R2/ΔK280. The distributions represent the probability of finding a conformation with a specific value of the end-to-end distance (Ree) and radius of gyration (Rg). R2/wt(a) shows more compact conformations (roughly the region with Ree < 1.5nm) than its mutant R2/ΔK280(b). On the other hand, when one chain within a dimer is considered for both R2/wt (c) and R2/ΔK280 (d), the distribution is shifted toward more extended conformations. Representative structures have been reported as well. See also figure S1 and S2 for how theoretical CCS have been computed.