Abstract
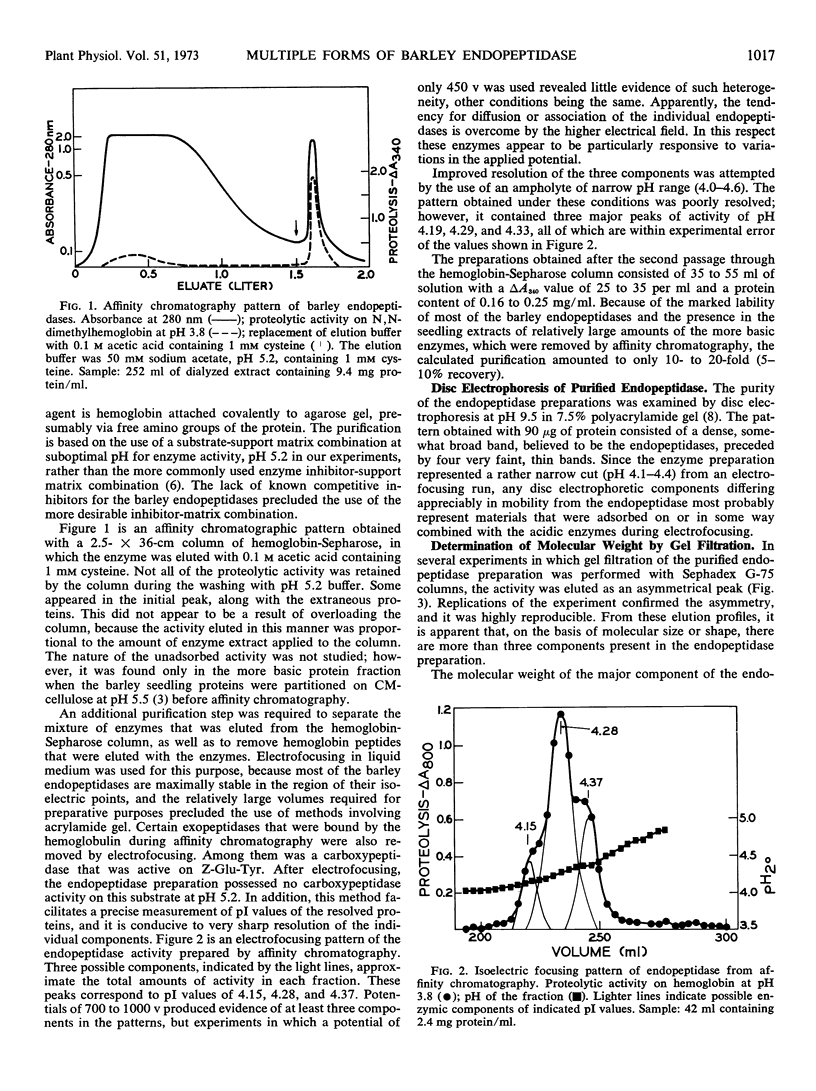
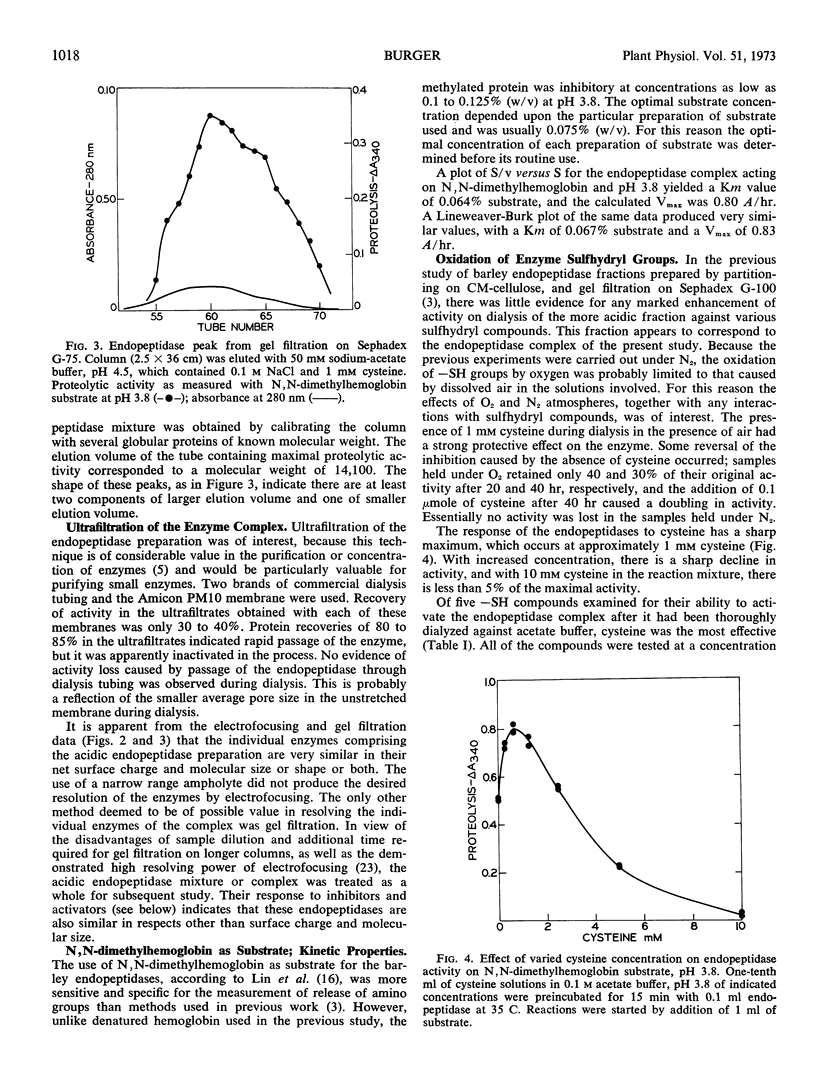
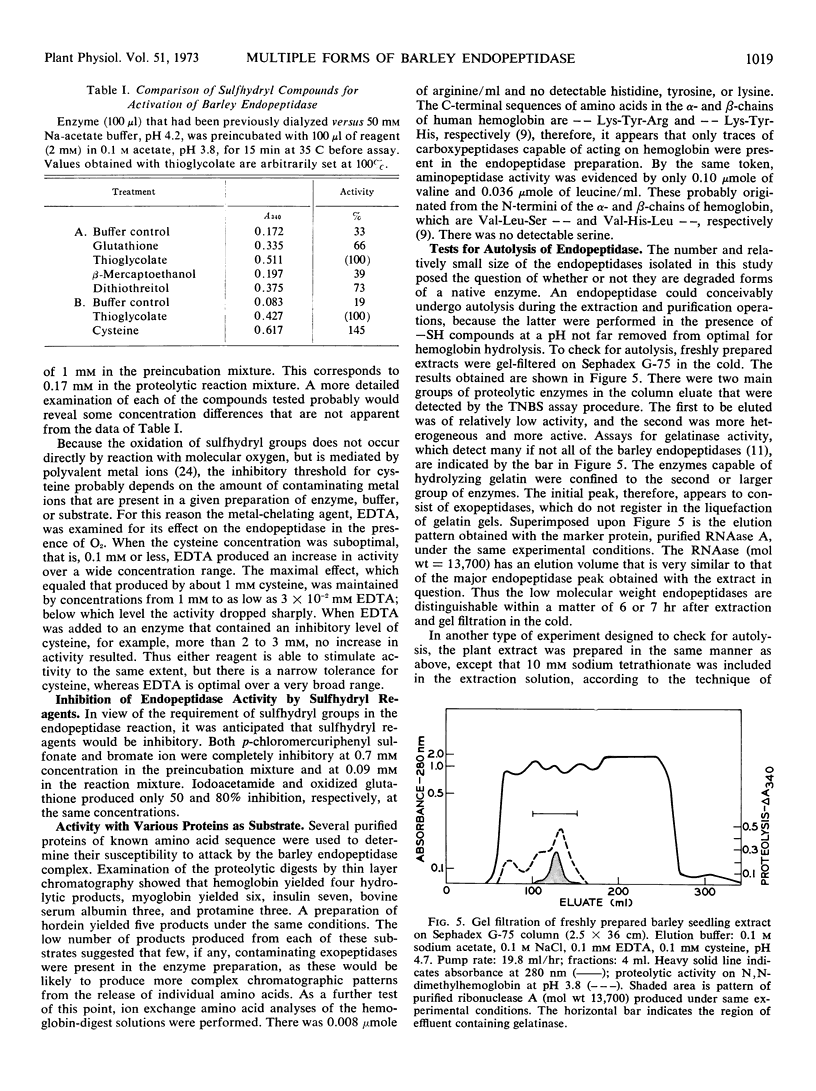
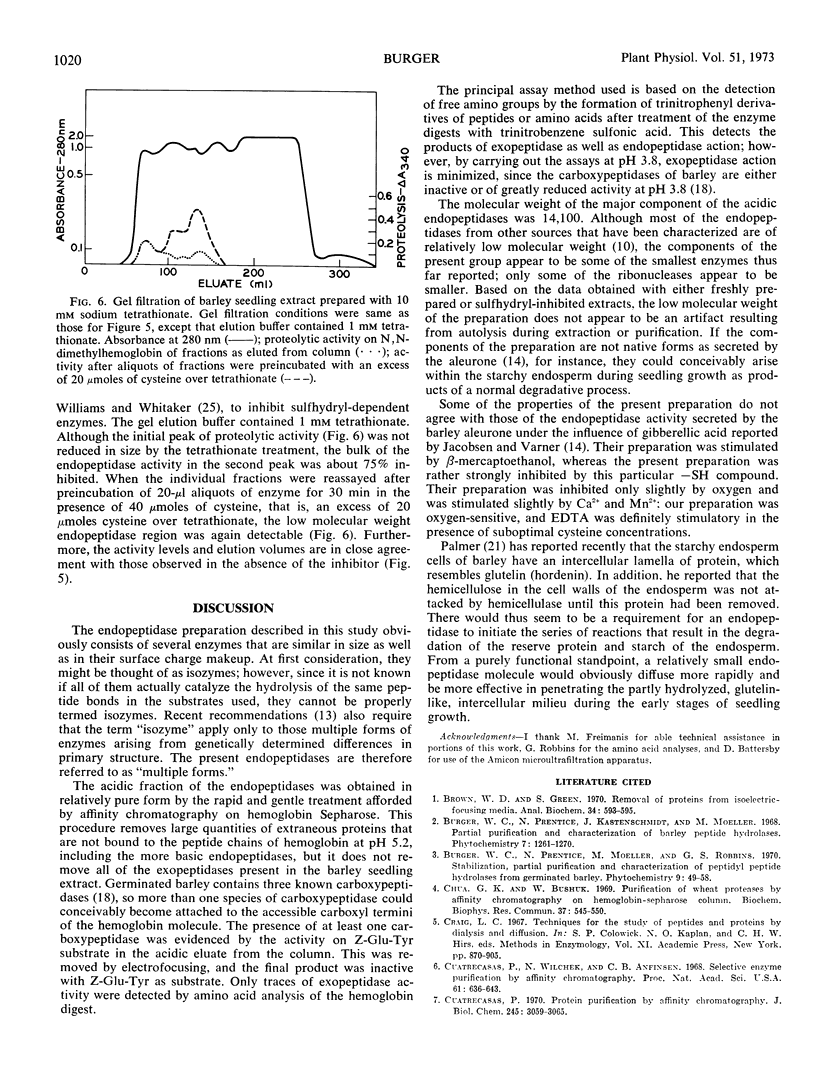
An endopeptidase preparation from germinated barley Hordeum vulgare L., cv. Trophy, purified by affinity chromatography and density-gradient electrofocusing, consisted of three or four components. The preparation was only partly resolved by electrofocusing, with evidence of three possible components (pI 4.15, 4.28, and 4.37). Gel filtration on Sephadex G-75 yielded an asymmetrical peak, the major part of which corresponded to a molecular weight of 14,100, with evidence of one larger and two smaller components. The activity of the preparation was sulfhydryl-dependent; cysteine was the most effective of several sulfhydryl compounds tested. The preparation was sensitive to O2 in the absence of metal chelating agents and was inhibited by sulfhydryl reagents. It showed very narrow concentration tolerances for both cysteine and a substrate, N,N-dimethylhemoglobin. The Km value on N,N-dimethylhemoglobin at pH 3.8 was 0.064 to 0.067% (w/v) substrate; Vmax was 0.80 to 0.83 A340 per hour. Normal enzyme activity and molecular-size distribution were observed when the endopeptidases were extracted in the inhibited state and subsequently reactivated, thus ruling out the possibility that the enzymes might be autolytic artifacts that arose during extraction and purification.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. D., Green S. Removal of proteins from isoelectric focusing media. Anal Biochem. 1970 Apr;34(2):593–595. doi: 10.1016/0003-2697(70)90145-4. [DOI] [PubMed] [Google Scholar]
- Chua G. K., Bushuk W. Purification of wheat proteases by affinity chromatography on hemoglobin-Sepharose column. Biochem Biophys Res Commun. 1969 Oct 22;37(3):545–550. doi: 10.1016/0006-291x(69)90950-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. V., Varner J. E. Gibberellic Acid-induced synthesis of protease by isolated aleurone layers of barley. Plant Physiol. 1967 Nov;42(11):1596–1600. doi: 10.1104/pp.42.11.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin Y., Means G. E., Feeney R. E. The action of proteolytic enzymes on N,N-dimethyl proteins. Basis for a microassay for proteolytic enzymes. J Biol Chem. 1969 Feb 10;244(3):789–793. [PubMed] [Google Scholar]
- Moeller M., Robbins G. S., Burger W. C., Prentice N. A carboxypeptidase from germinated barley and its action on casein. J Agric Food Chem. 1970 Sep-Oct;18(5):886–890. doi: 10.1021/jf60171a018. [DOI] [PubMed] [Google Scholar]
- Susor W. A., Kochman M., Rutter W. J. Heterogeneity of presumably homogeneous protein preparations. Science. 1969 Sep 19;165(3899):1260–1262. doi: 10.1126/science.165.3899.1260. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Whitaker J. R. Multiple Molecular Forms of Ficus glabrata Ficin. Their Separation and Relative Physical, Chemical, and Enzymatic Properties. Plant Physiol. 1969 Nov;44(11):1574–1583. doi: 10.1104/pp.44.11.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]



