Table 2.
Parameter estimates from the final population pharmacokinetic model
| Parameter | Value | RSE (%) | Description |
|---|---|---|---|
| Fixed effects | |||
| CL/F a (L/h) | 12.4 | 28.71 | Total body clearance (renal and nonrenal) |
| V 2/F (L) | 531 | 22.60 | Volume of distribution of the central compartment |
| Q/F (L/h) | 152 | 14.34 | Intercompartmental clearance |
| V 3/F (L) | 499 | 9.42 | Volume of distribution of the peripheral compartment |
| k a (h−1) | 0.821 | 16.81 | First-order absorption rate constant |
| ALAG (h) | 1.67 | 4.56 | Absorption lag time |
| ALAG_3rd (h) | 0b | – | Absorption lag time of the third dose (fasted) |
| F | 1.00b | – | Relative bioavailability |
| EC50 food time c (h) | 0.556 | 11.13 | Time between dose administration and food intake at which the effect on bioavailability is half of the maximum effect |
| F min food time c | 0b | – | Minimum bioavailability when time between dose administration and food intake is 0 (fixed to 0 due to limited data) |
| Hillfood time c | 6.10 | 48.52 | Hill factor describing the steepness of the relation between time to food intake and the relative bioavailability |
| K o A d (mL/min) | 313 | 23.39 | Hemodialyzer mass transfer-area coefficient |
| Random effects: interindividual variability (IIV) and interoccasion variability (IOV) | |||
| IIV CL/F (CV%) | 40.4 | 43.01 | IIV in the total body clearance |
| IIV V 2/F (CV%) | 14.3 | 43.07 | IIV in the apparent volume of distribution of the central compartment |
| IOV k a (CV%) | 64.0 | 30.24 | IOV in the relative first-order absorption rate constant |
| IOV F (CV%) | 48.0 | 26.91 | IOV in the relative bioavailability |
| Random effects: residual variability | |||
| PRV (CV%) | 8.5 | 24.00 | Proportional residual variability |
aCLtotal/F = CLdialysis/F + θCL/F × eηCL
bParameters fixed
cF3rd_dose = (θFmin food time + (1 − θFmin food time) × 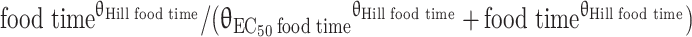 ) × eκF
) × eκF
dSee Eq. 1
CV coefficient of variation, RSE relative standard error, CL total/F total apparent dabigatran clearance, CL dialysis/F apparent dabigatran dialysis clearance, θ symbol for fixed-effect parameter estimate, η symbol for interindividual variability, F 3rd_dose relative bioavailability of the third dose in each period, κ symbol for interoccasion variability
