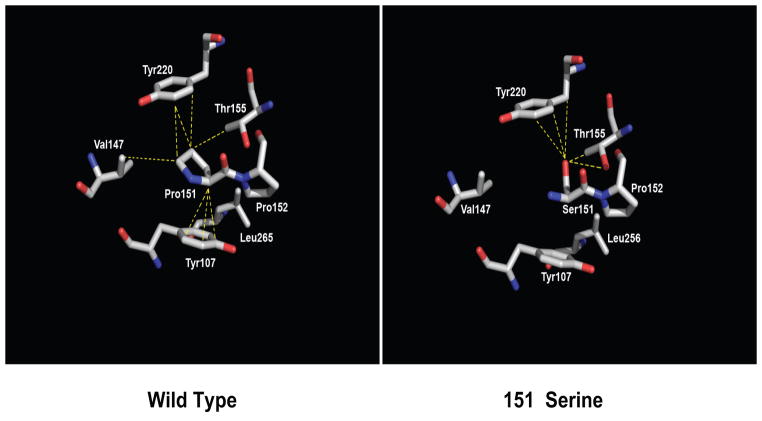
Figure 3.
p53P151S makes fewer inter residue contacts than wild type p53. (Left) Structure of wild type p53 in the vicinity of residue P151, Residue P151 and nearby interacting amino acid residues are shown as sticks and colored according to atom type whereby oxygen is red, nitrogen, blue and carbon, white. Dashed yellow lines indicate contacts between residue P151 and nearby residues (d ≤ 4.2 Å). (Right) The structure of the modeled p53P151S mutant, colored and demarked as in the left panel. The closest approach of the side chains of S151 and V147 is 5.8 Å.