Abstract
Purpose
We conducted a proteomic analysis of synovial fluid (SF) to identify differentially expressed proteins and analyse their correlation with osteoarthritis (OA) severity. Our primary purpose was to gain insight into the pathogenesis of OA.
Methods
SF samples were acquired from 12 knee OA patients and 12 non-OA controls (ten had a meniscus injury, two had a discoid meniscus and all exhibited intact articular cartilage) and sequentially subjected to two-dimensional electrophoresis (2-DE). The radiographic grading of knee OA was performed using the Kellgren-Lawrence criteria. Differentially expressed proteins were identified by matrix-assisted laser desorption/ionisation time-of-flight/time-of-flight mass spectrometry (MALDI-TOF/TOF MS). Proteins of interest identified from SF were detected using an enzyme-linked immunosorbent assay (ELISA).
Results
A total of 31 protein spots showed significant differences (p < 0.05) between the sample groups; 25 of the 31 spots (80.6 %) were identified as proteins of interest. Among them 20 corresponded to up-regulation and five to down-regulation in OA samples. HLA-DR was one of the proteins up-regulated, which was confirmed by ELISA.
Conclusions
These observations have implications in delineating the protein expression underlying the pathogenesis of OA and facilitate further elucidation of molecular mechanisms involved in disease progression. Substantial alterations of the protein profile in SF may be associated with OA severity.
Introduction
Osteoarthritis (OA) is the most common articular disease characterised by progressive degeneration of articular cartilage and secondary hyperplasia of osteophytes. According to an epidemiological survey, more than 8.9 % of the population suffer from clinically significant OA [1] which is the primary cause of pain and dysfunction in the joint. The pathogenesis of OA has not yet been completely elaborated. Most individuals are clinically asymptomatic in the early stage of OA. Visible pathological changes in cartilage generally pre-exist when symptoms arise. Nowadays, the diagnosis of OA mainly depends on the symptoms and radiographic manifestations which are limited in the early diagnosis. This limitation gives rise to the interest in proteomic studies to find candidate biomarkers for the evaluation of cartilage degeneration and disease severity, so as to realise early diagnosis and indicate the progression and prognosis of OA. Most importantly, proteomic studies also enable the pathogenesis of OA to be studied at the molecular level from a comprehensive perspective and shed light on the abnormal metabolic mechanism in the articular cartilage during the disease progression.
As is well known, there is an exchange of proteins between synovial fluid (SF) and the systemic circulation through the synovial lymphatics and vasculature [2]. However, SF is more than a filtrate from the systemic circulation; joint tissues including synovium, ligament, meniscus and joint capsule also secrete proteins into SF. Beyond that, the dynamic equilibrium between the continuous, ongoing formation and breakdown of the cartilaginous matrix would allow protein molecules synthesised by chondrocytes to be released into SF; the anabolism and catabolism of the extracellular matrix (ECM) are regulated by an interplay of metabolic factors in order to meet a balance in normal conditions. Altered synthesis of ECM proteins by chondrocytes and release of the products of cartilage ECM degradation give rise to proteomic changes in SF when the homeostasis is broken, finally leading to the formation of a cartilage ulcer. Consequently, proteins and protein fragments from cartilage and joint tissues in SF can be chosen as candidate biomarkers for OA. Compared with serum, the concentration of any protein from cartilage is higher in SF, the components of which are also less influenced by systematic conditions, thus it is more suitable to detect protein profiles in SF than in serum.
The introduction of proteomics techniques has enabled the evaluation of a large number of proteins in complex protein mixtures and quantitative comparisons of the alterations in protein profiles on a large scale. Wu et al. [3] applied a magnetic bead separation system to extract and analyse urinary peptides from IgA nephropathy patients to identify potential biomarkers for non-invasive diagnosis of this disease. Bead-based fractionation systems are a new type of multifunctional method developed in recent years that can selectively separate certain proteins and peptides according to different chemical chromatographic surfaces on the outer layer of magnetic beads for analysis using matrix-assisted laser desorption/ionisation time-of-flight/time-of-flight mass spectrometry (MALDI-TOF/TOF MS). We used this system to separate proteins and peptides in SF of OA patients in the preliminary stage of this research. However, the result was unsatisfactory for no peptide peak was detected in peptide mass fingerprinting (PMF), probably because this fractionation method is generally used to extract proteins or peptides that can uniquely chelate a magnetic bead and is sensitive for separating peptides with a low molecular weight (MW < 20 kDa), whereas the MWs of proteins in SF profile mostly range from 20 to 100 kDa according to the pertinent literature [2]. It is probably unsuitable to introduce a magnetic bead separation system to proteomics analysis of SF for the investigation of OA.
Recently, the combinative technique of two-dimensional electrophoresis (2-DE) and MS has been widely applied to screen for differentially expressed proteins. Lambrecht et al. [4] used this method to reveal substantial alterations in protein profiles of OA chondrocytes; similar studies [5, 6] describing the proteome by this technique have also been published. Using 2-DE combined with MS, we were able to identify proteins displaying an altered abundance in SF from OA. These observations may shed new light on the pathogenesis of OA and offer original insights into the molecular mechanisms involved in disease progression.
Materials and methods
Subjects
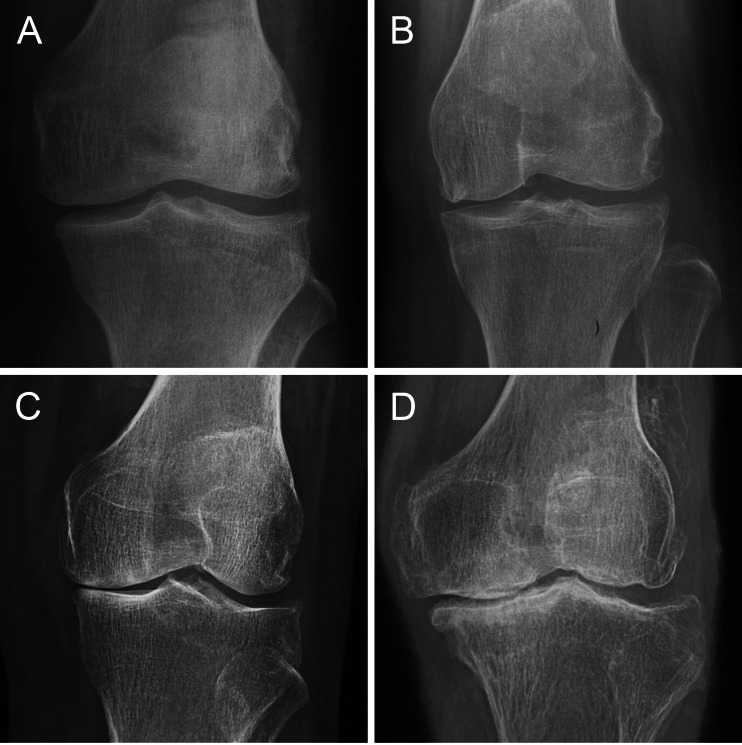
SF samples were acquired from 12 knee OA patients (six men and six women, age 44–70 years, mean age 53 years) and 12 non-OA controls (seven men and five women, age 28–54 years, mean age 39 years) with other knee disorders (meniscus injury or discoid meniscus without cartilage defect). All of the patients with meniscus injury recruited were examined arthroscopically between one and three days after injury. All of the subjects were chosen from patients who underwent arthroscopy or arthroplasty for treatment of a knee disorder between August 2011 and April 2012 in the Department of Orthopaedics of PLA General Hospital and identified by the medical record number they were given when they were hospitalised. The diagnosis of knee OA was based on the criteria of the American College of Rheumatology (ACR). All of the subjects signed informed consent documents. The research was approved by the Institutional Review Board (IRB). The severity of knee OA was evaluated by the Kellgren-Lawrence (KL) radiographic grading criteria (Fig. 1) [7], according to which seven OA patients recruited in the research were classified as grade 4, and the other five were categorised as grade 2.
Fig. 1.
The Kellgren-Lawrence radiographic grading criteria. a Grade 1, doubtful narrowing of joint space and possible osteophytic lipping. b Grade 2, definite osteophytes and possible narrowing of joint space. c Grade 3, moderate multiple osteophytes, definite narrowing of joints space, some sclerosis and possible deformity of bone contour. d Grade 4, large osteophytes, marked narrowing of joint space, severe sclerosis and definite deformity of bone contour
Sample collection and preparation
Prior to surgery, 1–2 ml of SF sample was aspirated from the affected knee with a sterile puncture needle and 50 μl of proteinase inhibitor (cocktail, Roche) was immediately added to the sample after collection. The mixture was then centrifuged at 8,000 rpm for 30 minutes at 4 °C (Hettich MIKRO 22R, Tuttlingen, Germany) to remove cells and debris and concentrate the proteins. After centrifugation, the SF sample was subjected to protein quantification using the Bradford Assay [8] with a commercial Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA) to determine the volume of sample to be loaded in 2-DE. Then the processed samples were stored at −80 °C until use.
Two-dimensional electrophoresis
Two-dimensional electrophoresis analyses were performed according to Cui et al. [9] with some modifications; 160 μg of protein was diluted to a volume of 350 μl with rehydration solution containing 8 M urea, 0.02 % 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulphonate (CHAPS) (Sigma), 0.02 M dithiothreitol (DTT), 0.05 % IPG buffer (Amersham Pharmacia Biotech) and loaded onto 18 cm (pH 3–10) non-linear immobilised pH gradient DryStrips (Amersham Pharmacia Biotech). Then, 800 μl of cover fluid was added to cover the DryStrips. Isoelectric focusing (IEF) was carried out using the IPGphor system (Amersham Pharmacia Biotech) under a constant voltage of 30 V/gel for the initial ten hours, followed by 500 V/gel for one hour, 1,000 V/gel for one hour and 8,000 V/gel for the last ten hours. After IEF, the strips were equilibrated in a solution containing 20 mM DTT, 50 mM Tris(hydroxymethyl)aminomethane (Tris-Cl), pH 8.8, 30 % glycerol, 6 M urea, 2 % sodium dodecyl sulphonate (SDS) and bromophenol blue for 15 minutes. A second equilibration step was performed in the same solution except for DTT, which was replaced by 100 mM iodoacetamide. Then, the IPG strips were transferred to vertical self-cast 13 % SDS polyacrylamide gels [13 % acrylamide, 375 mM Tris-Cl, 0.1 % SDS, 0.1 % ammonium persulphate (APS), tetramethylethylenediamine (TEMED)] and embedded with agarose sealing solution (0.5 % agarose, SDS electrode buffer, bromophenol blue). Finally, SDS polyacrylamide gel electrophoresis (SDS-PAGE) was carried out using the PROTEAN II xi Cell vertical electrophoresis system (Bio-Rad Laboratories) under a constant current of 20 mA/gel for the initial 40 minutes and 30 mA/gel thereafter until the bromophenol blue dye marker reached the bottom of the gel. Samples from the same patient were run at least two times to determine the variability.
Protein spot visualisation and 2-DE image analysis
Silver nitrate staining was performed in gels according to the protocol of Mortz et al. [10]. An ImageScanner (Amersham Pharmacia Biotech) was used to capture 2-DE images. ImageMaster 2D Platinum (Amersham Pharmacia Biotech) was applied to quantify and revise the intensity of protein spots. Spot intensity was normalised for every gel [(spot quantity)/∑(spot quantities)] to correct for subtle variations in sample loading and gel staining between the gels.
In-gel digestion and peptide extraction
After analysing 2-DE images, in-gel digestion was performed using a protocol previously described by Shevchenko et al. [11]
Mass spectrometry (MS) and data interpretation
MALDI-TOF/TOF MS was performed in a Bruker UltraflexTM III MALDI-TOF/TOF MS (Bruker Daltonics) according to a published protocol [12]. The obtained peptide mass fingerprints (PMFs) were used to search against the Shigella flexneri 2a 2457T database using the program Mascot 2.1 (Matrix Science Ltd.) to eliminate redundancy resulting from multiple members of the same protein family, and the results were checked against the NCBInr database (version 21 October 2006, 4,072,503 sequences).
Enzyme-linked immunosorbent assay (ELISA)
Another 57 SF samples were obtained from 45 knee OA patients (21 men and 24 women, age 40–72 years mean age: 51 years) and 12 non-OA controls (seven men and five women, age 26–49 years, mean age 37 years) to detect the HLA-DR level. Among the 45 OA patients, 18 were classified as grade 2 according to the KL grading criteria, while 17 were grade 3 and ten were grade 4. The HLA-DR level in SF was measured by ELISA (MyBioSource, R&D Systems, San Diego, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with SPSS 13.0 for Windows. Data were expressed as a mean ± SD. Comparisons between two groups were performed using Student’s t test. The significance of differences among the non-OA group and OA subgroups of different severity was determined by analysis of variance (ANOVA). Pearson’s correlation coefficient was used to analyse the correlation between protein level and OA severity. A p value < 0.05 was considered statistically significant.
Results
Two-dimensional electrophoresis image analysis
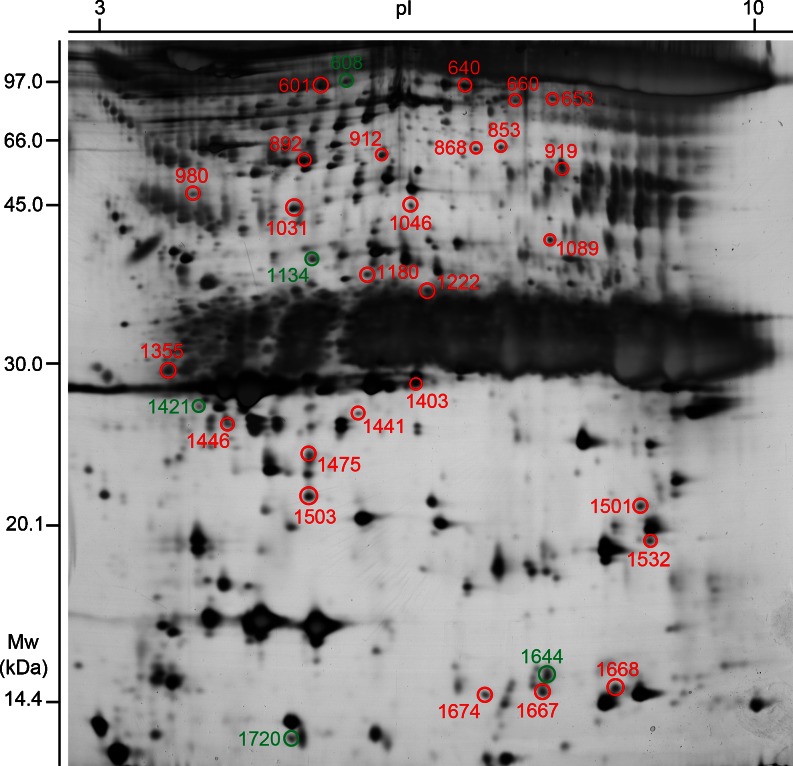
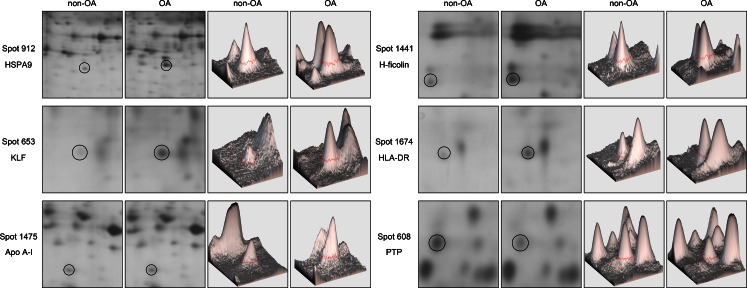
The mean total protein concentration in SF was 24.67 ± 2.85 μg/μl. A total of 682 ± 35 protein spots could be detected on each gel. Protein spots were regarded to be differentially expressed when spot intensity between two groups was proven to be significantly different (p < 0.05). Based on these criteria, the comparison between OA and non-OA groups resulted in the identification of 31 significantly different protein spots, with molecular weights (MWs) ranging from 14 kDa to 97 kDa. These spots were excised manually from gels and identified by MS. A total of 25 spots of the 31 excised (80.6 %) were identified as proteins of interest. Among them 20 were up-regulated and five were down-regulated in OA samples compared with control samples (Fig. 2). Figure 3 shows close-up sections and ImageMaster 3D views of six differentially expressed protein spots in SF from OA patients. These data revealed that a large number of proteins showed statistically significant differences in spot intensity between the two groups.
Fig. 2.
A 2-DE image of SF proteins. Spots showing statistically different abundance in OA are enclosed in circles and numbered according to spot ID. Spots in red circles correspond to up-regulation; in contrast, those in green circles refer to down-regulation
Fig. 3.
Close-up sections and ImageMaster 3D views of six differentially expressed protein spots in SF from OA patients. HSPA9 human heat shock 70 kDa protein 9, KLF Krüppel-like zinc finger protein, Apo A-I apolipoprotein A-I, HLA-DR human leucocyte antigen DR, PTP protein tyrosine phosphatase
Protein identification by MS
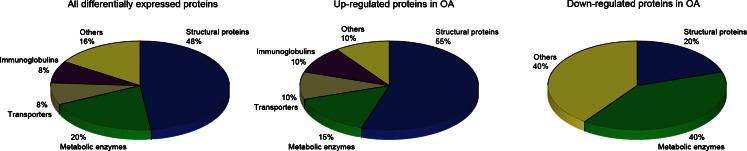
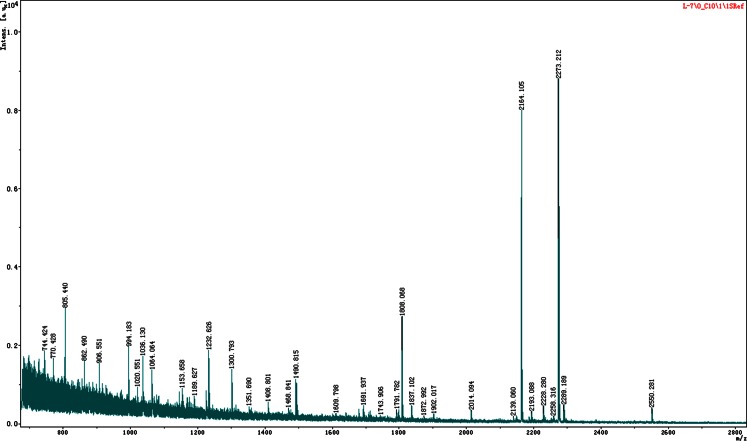
Protein identification results are shown in Table 1. A total of 25 different proteins were identified as proteins of interest. Keratin (spot 980) and albumins (spots 1222, 1355, 892, 1532 and 1446) were considered as meaningless. According to their biological function, proteins listed in Table 1 were classified into the following categories: structural proteins (12 proteins, 48 %), metabolic enzymes (five proteins, 20 %), transporters (two proteins, 8 %), immunoglobulins (two proteins, 8 %) and others (four proteins, 16 %). Figure 4 depicts the distribution of differential proteins according to functional categories. Figure 5 shows the PMF analysed by MALDI-TOF/TOF MS on the protein spot 1674 (HLA-DR).
Table 1.
The differentially expressed proteins between OA and non-OA groups identified by MALDI-TOF/TOF MS
| Spot ID | Protein name | Mr | pI | Peptides | Sequence coverage | Score | Protein expression | |
|---|---|---|---|---|---|---|---|---|
| Match | Total | |||||||
| 1031 | Apolipoprotein A-IV | 45,353 | 5.33 | 19 | 79 | 38 % | 92 | ↑ |
| 608 | Protein tyrosine phosphatase (PTP) | 135,617 | 5.91 | 27 | 83 | 24 % | 77 | ↓ |
| 640 | Dynamin | 97,545 | 7.31 | 20 | 69 | 22 % | 75 | ↑ |
| 1503 | HLA-B α chain | 21,063 | 5.97 | 21 | 60 | 47 % | 86 | ↑ |
| 1046 | Immunoglobulin heavy chain constant region | 49,433 | 6.40 | 8 | 47 | 17 % | 98 | ↑ |
| 1668 | Chain A, solution structure of the mitochondrial ribosomal protein L17 isologue | 13,656 | 9.37 | 10 | 61 | 73 % | 68 | ↑ |
| 919 | RNA binding protein, homologue 2 | 60,515 | 9.56 | 13 | 58 | 27 % | 81 | ↑ |
| 853 | A kinase (PRKA) anchor protein 4 | 75,598 | 8.48 | 17 | 71 | 29 % | 87 | ↑ |
| 1644 | Chain A, crystal structure of mutant human lysozyme | 14,606 | 9.14 | 7 | 65 | 45 % | 76 | ↓ |
| 1180 | Hakata antigen | 32,868 | 6.20 | 10 | 64 | 32 % | 74 | ↑ |
| 1441 | H-ficolin | 25,068 | 6.14 | 8 | 50 | 34 % | 66 | ↑ |
| 660 | Microtubule-associated protein 1B | 81,909 | 8.80 | 15 | 72 | 20 % | 73 | ↑ |
| 1667 | Immunoglobulin ε heavy chain variable region | 11,563 | 9.01 | 6 | 56 | 70 % | 96 | ↑ |
| 601 | Axonemal dynein light chain domain-containing protein 1 | 117,953 | 5.49 | 19 | 67 | 21 % | 75 | ↑ |
| 868 | PAB-dependent poly(A)-specific ribonuclease subunit 3 | 61,990 | 8.29 | 12 | 74 | 29 % | 84 | ↑ |
| 653 | Krüppel-like zinc finger protein (KLF) | 75,080 | 9.44 | 10 | 45 | 20 % | 82 | ↑ |
| 1421 | Calumenin isoform 5 | 26,847 | 4.61 | 6 | 40 | 31 % | 70 | ↓ |
| 1403 | Scavenger receptor | 28,852 | 6.65 | 8 | 55 | 29 % | 74 | ↑ |
| 1475 | Apolipoprotein A-I | 23,389 | 5.55 | 7 | 31 | 35 % | 127 | ↑ |
| 1134 | Secernin | 41,237 | 5.80 | 7 | 48 | 21 % | 69 | ↓ |
| 1720 | β-catenin-interacting protein 1 | 9,165 | 5.33 | 5 | 36 | 59 % | 66 | ↓ |
| 1674 | HLA-DR | 10,953 | 6.08 | 4 | 54 | 43 % | 87 | ↑ |
| 912 | HSPA9 protein | 73,808 | 6.04 | 16 | 57 | 34 % | 82 | ↑ |
| 1501 | Ribosomal protein L27a (RPL27a) | 3,706 | 9.69 | 3 | 21 | 82 % | 75 | ↑ |
| 1089 | Nucleolar phosphoprotein p130 (p130) | 73,677 | 9.48 | 7 | 39 | 16 % | 85 | ↑ |
The spot ID refers to the number used in 2-DE to indicate the location of the protein. The protein score is −10 × Log(P), where P is the probability that the observed match is a random event. Protein scores greater than 66 are significant (p < 0.05). “↑”indicates the up-regulated proteins in OA. “↓”indicates the down-regulated proteins in OA
Fig. 4.
Graphical representation of the functional classification of differentially expressed proteins in SF from OA patients compared with non-OA controls based on the data in Table 1
Fig. 5.
PMF of spot 1674 by MALDI-TOF/TOF MS. After a database search, the protein was identified as HLA-DR. The protein score (87) is significant (p < 0.05) and sequence coverage reaches 43 %
ELISA of HLA-DR
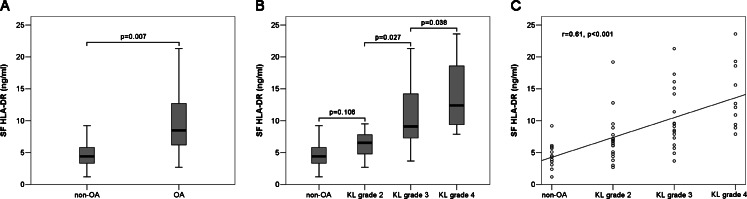
As is shown in Fig. 6, the mean level of HLA-DR in SF from OA patients (9.86 ± 5.17 ng/ml) is higher than that from non-OA patients (4.58 ± 2.06 ng/ml), and the difference is statistically significant (p = 0.007). According to the KL grading criteria, the mean level of HLA-DR in SF from KL grade 2 is 7.14 ± 3.87 ng/ml, that from KL grade 3 is 10.36 ± 4.86 ng/ml and that from KL grade 4 is 13.90 ± 5.20 ng/ml. It was found that the mean level of HLA-DR in SF from KL grade 4 is significantly elevated compared with those from non-OA patients (p < 0.001), KL grade 2 (p < 0.001) and KL grade 3 (p = 0.038). Moreover, the mean level of HLA-DR in SF from KL grade 3 is markedly higher than those from non-OA patients (p = 0.001) and KL grade 2 (p = 0.027). Although the mean level of HLA-DR in SF from KL grade 2 is greater than that from non-OA patients, the difference was not statistically significant (p = 0.106). The levels of HLA-DR in SF are positively correlated with OA severity (r = 0.61, p < 0.001).
Fig. 6.
ELISA results. a, b Box plot presentation of HLA-DR concentrations in SF from non-OA and OA patients. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers below and above the box indicate the minimal and the maximal values recorded, respectively. c Scatter plot presentation of the relationship between HLA-DR concentrations and OA severity. The plot shows that they are positively correlated (r = 0.61, p < 0.001)
Discussion
OA is characterised by the phenomena of synovial hyperplasia and hyperosteogeny which can be reflected in our analysis by the identification of proteins involved in cell proliferation. These proteins and their possible mechanism in proliferation are stated as follows: (1) KLF10, also known as transforming growth factor-β-inducible early gene (TIEG1), is a member of the Krüppel family of transcription factors in human osteoblasts [13], playing a pleiotropic role in cell proliferation, endocrine, metabolism and circadian signals [14]. It regulates the expression and activity of Runx2 which is known to be a determining transcription regulator of cell phenotype commitment and growth [15] and essential for osteoblast lineage commitment, differentiation, bone matrix formation and mineralisation [16]. The positive regulation of KLF10 in Runx2 expression implies its additional role in regulating osteoblastogenesis and bone development [17]. (2) p130 [18] and PTP were reported to be expressed in response to cell growth signals and tightly associated with cell proliferation. As a GTP/ATP binding protein with intrinsic GTPase/ATPase activities, the alterations of p130 during mitosis are well correlated with the nucleolar disassembly and reassembly [19]. (3) As for HSPA9, a mitochondrial chaperone of the heat shock protein (HSP) 70 family, it has important roles in stress response, glucose regulation, cell proliferation, differentiation and tumourigenesis. HSPA9 overexpression can suppress the pro-apoptotic effect of various substances [20]. (4) RPL27a is required to maintain the growth rate in higher eukaryotes; the loss of RPL27a can result in a reduced growth rate as well as developmental defects [21].
Besides the above-mentioned proteins described to promote cell proliferation, there are also some proteins which may play a suppressive role. In previous reports in the literature, high secernin-1 expression seemed to be correlated with better prognosis in synovial sarcoma patients compared to those with low expression of secernin-1 [22]. Another down-regulated protein identified in our analysis, β-catenin-interacting protein, can inhibit the Wnt signalling pathway to mediate cell growth, proliferation and tumourigenesis [23]. These proteins may work together and mediate cell proliferation during OA progression.
Inflammatory arthritis patients always have higher SF apolipoprotein levels, reflecting increased synovial permeability due to vasodilatation from pre-existing blood vessels or to marked neoangiogenesis [24]. SF apolipoproteins can modulate the inflammatory reaction within the synovial space. Infiltration of apolipoprotein A-I has been observed in rheumatoid arthritis (RA) patients in the perivascular regions of synovial tissue where it can locally inhibit the production of proinflammatory cytokines by monocyte/macrophages upon direct contact with stimulated T lymphocytes [25]. In addition, increased apolipoprotein A-1 levels are also associated with knee OA [26]. Recently it has been shown to influence the immune response by modulating the Treg response [27]. Besides apolipoproteins, the high-density lipoprotein (HDL) receptor scavenger receptor class B, type 1 (SR-B1) expressed in RA synovial tissue can mediate acute phase serum amyloid A (A-SAA)-induced proinflammatory pathways [28]. The increase of these proteins in SF suggests that an inflammatory mechanism may also be involved in the pathogenesis of OA.
In recent years, more and more studies have attached importance to the immunological mechanism in OA pathogenesis. Cooke detected the precipitation of anti-collagen II IgG, IgA and complement C3 on the surface of deficient articular cartilage [29]. This coincides with the identification of immunoglobulin heavy chain constant and variable region in our study. In addition to humoral immunity, cellular immunity may also be considered as a potential determinant involved in OA pathogenesis. This can be implied in two proteins identified in our analysis. HLA-B is strongly associated with predisposition to rheumatoid and other autoimmune disorders. Like other MHC-I molecules, it is involved in the cellular immune reactivity of cytolytic T lymphocytes. Compared to HLA-B, the increase of soluble HLA-DR (sHLA-DR) has been verified by ELISA in our study; moreover, SF HLA-DR levels show positive correlation with OA severity. As was demonstrated in previous reports in the literature, the shared epitope referring to the conserved linear sequence of amino acids in the DRB1 chain of the HLA-DR α/β heterodimer was associated with joint destruction and periodontal disease in RA [30]. In addition, a protective effect of DERAA-containing HLA-DRB1 alleles on RA development has been previously reported [31]. Elevated SF sHLA-DR may be related to various HLA-DR turnover rates or differently regulated release mechanisms, allele-specific “secretor types”, and proteolytic cleavage of cell-bound counterparts may also contribute to the increase [32]. HLA-DR may modulate T-cell activation in at least two ways: On the one hand, sHLA-DR may suppress T-cell activation by competing with their cell-bound counterparts for TCR and/or CD4 binding. The appearance of smaller sized sHLA-DR may be an attempt of the immune system to counteract the expansion of CD4 T lymphocytes as a result of the disease [32]. On the other hand, autologous sHLA-DR presented by antigen-presenting cells (APCs) can trigger a vicious circle of immunostimulation and promote the spontaneous proliferation of SF monocytes in a manner analogous to the autologous mixed lymphocyte reaction under pathological conditions [33]. Such HLA-DR driven, macrophage/T cell interactions occurring in the synovial membrane are thought to initiate RA in the absence of exogenous antigens [34]. In summary, different forms of sHLA-DR may exert contrary (tolerogenic or immunogenic) effects.
The hypothesis that the complement system may participate in OA pathogenesis can be substantiated by the identification of H-ficolin/Hakata antigen. Ficolins are soluble pattern recognition molecules that activate the complement system via the lectin pathway [35]. One study has described an association between two single-nucleotide polymorphisms in the ficolin gene and RA susceptibility [36]. In addition, ficolin in SF is correlated with the total number of leucocytes and polynuclear cells in SF and plasma C-reactive protein (CRP) in RA patients [37]. Thus, it can be inferred that an immunological mechanism including humoral and cellular immunity along with the complement system may be a non-neglectable determinant contributing to OA pathogenesis.
To compare the SF protein expression between OA and healthy people will further elucidate the molecular mechanism of OA and find potential new markers for OA diagnosis. In our study, we first intended to include healthy individuals as a control group but this could not be realised due to ethical restrictions. However, we consider that it is reasonable to choose non-OA patients with other specific knee disorders such as meniscus injury or discoid meniscus as the control group. The reasons are stated as follows: (1) Considering that protein expression in SF may depend on the time course after injury, we examined the patients with meniscus injury recruited in our study between one and three days after injury and aspirated SF simultaneously; as far as we know, SF protein expression cannot change obviously in a short time after injury. In addition, the period is also too short to generate proteins derived from cartilage matrix degradation in secondary articular cartilage injury or proteins having a reparative effect on secondary tissue lesions. The phenomenon that most differentially expressed proteins detected in our study is due to promotion of proliferation or an immunological mechanism rather than trauma or acute inflammation can further prove this opinion. We are, thus, able to say that, except for some acute phase proteins, there could not be much difference in the SF protein spectrum of patients with meniscus injury compared to that of healthy people. (2) Articular cartilage is the main target of the harmful influences that cause OA and the structure in which the main pathological changes occur. Nevertheless, all of the patients with meniscus injury chosen as controls exhibited intact articular cartilage under arthroscopy. (3) None of the meniscus injuries in controls involved the red zone which is rich in blood vessels, so we can also exclude the possibility of blood contamination. For the reasons given above, we considered non-OA patients fulfilling these conditions as an appropriate control group in this scenario.
Many proteins identified are reported to be differently expressed in SF from OA patients for the first time in our analysis; some of them have been reported to be involved in the pathogenesis of other inflammatory arthritides, which might also play a potential role in OA. However, for lack of corresponding functional analysis of these differentially expressed proteins, we do not exactly know how these proteins act in OA progression, which will need further research.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107(9):152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers SL. Synovial fluid markers in osteoarthritis. Rheum Dis Clin North Am. 1999;25(2):433–449. doi: 10.1016/S0889-857X(05)70077-6. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Wang N, Wang J, Xie Y, Li Y, Liang T, Yin Z, He K, Chen X. Identification of a uromodulin fragment for diagnosis of IgA nephropathy. Rapid Commun Mass Spectrom. 2010;24(14):1971–1978. doi: 10.1002/rcm.4601. [DOI] [PubMed] [Google Scholar]
- 4.Lambrecht S, Verbruggen G, Verdonk PC, Elewaut D, Deforce D. Differential proteome analysis of normal and osteoarthritic chondrocytes reveals distortion of vimentin network in osteoarthritis. Osteoarthritis Cartilage. 2008;16(2):163–173. doi: 10.1016/j.joca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Hermansson M, Sawaji Y, Bolton M, Alexander S, Wallace A, Begum S, Wait R, Saklatvala J. Proteomic analysis of articular cartilage shows increased type II collagen synthesis in osteoarthritis and expression of inhibin betaA (activin A), a regulatory molecule for chondrocytes. J Biol Chem. 2004;279(42):43514–43521. doi: 10.1074/jbc.M407041200. [DOI] [PubMed] [Google Scholar]
- 6.Smith MA, Bains SK, Betts JC, Choy EHS, Zanders ED. Use of two-dimensional gel electrophoresis to measure changes in synovial fluid proteins from patients with rheumatoid arthritis treated with antibody to CD4. Clin Diagn Lab Immunol. 2001;8(1):105–111. doi: 10.1128/CDLI.8.1.105-111.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramagli LS. Quantifying protein in 2-D PAGE solubilization buffers. Methods Mol Biol. 1999;112:99–103. doi: 10.1385/1-59259-584-7:99. [DOI] [PubMed] [Google Scholar]
- 9.Cui JW, Wang J, He K, Jin BF, Wang HX, Li W, Kang LH, Hu MR, Li HY, Yu M, Shen BF, Wang GJ, Zhang XM. Proteomic analysis of human acute leukemia cells: insight into their classification. Clin Cancer Res. 2004;10(20):6887–6896. doi: 10.1158/1078-0432.CCR-04-0307. [DOI] [PubMed] [Google Scholar]
- 10.Mortz E, Krogh TN, Vorum H, Görg A. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics. 2001;1(11):1359–1363. doi: 10.1002/1615-9861(200111)1:11<1359::AID-PROT1359>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1(6):2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 12.Zhu L, Zhao G, Stein R, Zheng X, Hu W, Shang N, Bu X, Liu X, Wang J, Feng E, Wang B, Zhang X, Ye Q, Huang P, Zeng M, Wang H. The proteome of Shigella flexneri 2a 2457T grown at 30 and 37 degrees C. Mol Cell Proteomics. 2010;9(6):1209–1220. doi: 10.1074/mcp.M900446-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spencer LT, Paone G, Krein PM, Rouhani FN, Rivera-Nieves J, Brantly ML. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am J Physiol Lung Cell Mol Physiol. 2004;286(3):L514–L520. doi: 10.1152/ajplung.00099.2003. [DOI] [PubMed] [Google Scholar]
- 14.Koczulla R, Jonigk D, Wolf T, Herr C, Noeske S, Klepetko W, Vogelmeier C, von Neuhoff N, Rische J, Wrenger S, Golpon H, Voswinckel R, Luisetti M, Ferrarotti I, Welte T, Janciauskiene S. Krüppel-like zinc finger proteins in end-stage COPD lungs with and without severe alpha1-antitrypsin deficiency. Orphanet J Rare Dis. 2012;7(1):29. doi: 10.1186/1750-1172-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, Choi JY, Komori T, Stein JL, Lian JB, Stein GS, van Wijnen AJ. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003;63(17):5357–5362. [PubMed] [Google Scholar]
- 16.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7(1–2):1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 17.Hawse JR, Cicek M, Grygo SB, Bruinsma ES, Rajamannan NM, van Wijnen AJ, Lian JB, Stein GS, Oursler MJ, Subramaniam M, Spelsberg TC. TIEG1/KLF10 modulates Runx2 expression and activity in osteoblasts. PLoS One. 2011;6(4):e19429. doi: 10.1371/journal.pone.0019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pai CY, Yeh NH. Cell proliferation-dependent expression of two isoforms of the nucleolar phosphoprotein p130. Biochem Biophys Res Commun. 1996;221(3):581–587. doi: 10.1006/bbrc.1996.0639. [DOI] [PubMed] [Google Scholar]
- 19.Chen HK, Yeh NH. The nucleolar phosphoprotein P130 is a GTPase/ATPase with intrinsic property to form large complexes triggered by F- and Mg2+ Biochem Biophys Res Commun. 1997;230(2):370–375. doi: 10.1006/bbrc.1996.5966. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, Morimoto RI. Cloning and subcellular localization of human mitochondrial hsp70. J Biol Chem. 1995;270(4):1705–1710. doi: 10.1074/jbc.270.4.1705. [DOI] [PubMed] [Google Scholar]
- 21.Szakonyi D, Byrne ME. Ribosomal protein L27a is required for growth and patterning in Arabidopsis thaliana. Plant J. 2011;65(2):269–281. doi: 10.1111/j.1365-313X.2010.04422.x. [DOI] [PubMed] [Google Scholar]
- 22.Suehara Y, Tochigi N, Kubota D, Kikuta K, Nakayama R, Seki K, Yoshida A, Ichikawa H, Hasegawa T, Kaneko K, Chuman H, Beppu Y, Kawai A, Kondo T. Secernin-1 as a novel prognostic biomarker candidate of synovial sarcoma revealed by proteomics. J Proteomics. 2011;74(6):829–842. doi: 10.1016/j.jprot.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H, Akiyama T. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes Dev. 2000;14(14):1741–1749. [PMC free article] [PubMed] [Google Scholar]
- 24.Oliviero F, Lo Nigro A, Bernardi D, Giunco S, Baldo G, Scanu A, Sfriso P, Ramonda R, Plebani M, Punzi L. A comparative study of serum and synovial fluid lipoprotein levels in patients with various arthritides. Clin Chim Acta. 2012;413:303–307. doi: 10.1016/j.cca.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Hyka N, Dayer JM, Modoux C, Kohno T, Edwards CK, 3rd, Roux-Lombard P, Burger D. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97(8):2381–2389. doi: 10.1182/blood.V97.8.2381. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Enríquez S, Torres-Carrillo NM, Vázquez-Del Mercado M, Salgado-Goytia L, Rangel-Villalobos H, Muñoz-Valle JF. Increase levels of apo-A1 and apo B are associated in knee osteoarthritis: lack of association with VEGF-460 T/C and +405 C/G polymorphisms. Rheumatol Int. 2008;29(1):63–68. doi: 10.1007/s00296-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 27.Wilhelm AJ, Zabalawi M, Owen JS, Shah D, Grayson JM, Major AS, Bhat S, Gibbs DP, Jr, Thomas MJ, Sorci-Thomas MG. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr−/−, ApoA-I−/− mice. J Biol Chem. 2010;285(46):36158–36169. doi: 10.1074/jbc.M110.134130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullan RH, McCormick J, Connolly M, Bresnihan B, Veale DJ, Fearon U. A role for the high-density lipoprotein receptor SR-B1 in synovial inflammation via serum amyloid-A. Am J Pathol. 2010;176(4):1999–2008. doi: 10.2353/ajpath.2010.090014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooke TD. Immune pathology in polyarticular osteoarthritis. Clin Orthop Relat Res. 1986;213:41–49. [PubMed] [Google Scholar]
- 30.Marotte H, Farge P, Gaudin P, Alexandre C, Mougin B, Miossec P. The association between periodontal disease and joint destruction in rheumatoid arthritis extends the link between the HLA-DR shared epitope and severity of bone destruction. Ann Rheum Dis. 2006;65(7):905–909. doi: 10.1136/ard.2005.036913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feitsma AL, Worthington J, van der Helm-van Mil AH, Plant D, Thomson W, Ursum J, van Schaardenburg D, van der Horst-Bruinsma IE, van Rood JJ, Huizinga TW, Toes RE, de Vries RR. Protective effect of noninherited maternal HLA-DR antigens on rheumatoid arthritis development. Proc Natl Acad Sci U S A. 2007;104(50):19966–19970. doi: 10.1073/pnas.0710260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claus R, Bittorf T, Walzel H, Brock J, Uhde R, Meiske D, Schulz U, Hobusch D, Schumacher K, Witt M, Bartel F, Hausmann S. High concentration of soluble HLA-DR in the synovial fluid: generation and significance in “rheumatoid-like” inflammatory joint diseases. Cell Immunol. 2000;206(2):85–100. doi: 10.1006/cimm.2000.1729. [DOI] [PubMed] [Google Scholar]
- 33.Duke O, Gordon Y, Panayi GS. Synovial fluid mononuclear cells exhibit a spontaneous HLA-DR driven proliferative response. Clin Exp Immunol. 1987;70(1):10–17. [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas R, Lipsky PE. Presentation of self peptides by dendritic cells: possible implications for the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1996;39(2):183–190. doi: 10.1002/art.1780390202. [DOI] [PubMed] [Google Scholar]
- 35.Thiel S, Gadjeva M. Humoral pattern recognition molecules: mannan-binding lectin and ficolins. Adv Exp Med Biol. 2009;653:58–73. doi: 10.1007/978-1-4419-0901-5_5. [DOI] [PubMed] [Google Scholar]
- 36.Vander Cruyssen B, Nuytinck L, Boullart L, Elewaut D, Waegeman W, Van Thielen M, De Meester E, Lebeer K, Rossau R, De Keyser F. Polymorphisms in the ficolin 1 gene (FCN1) are associated with susceptibility to the development of rheumatoid arthritis. Rheumatology (Oxford) 2007;46(12):1792–1795. doi: 10.1093/rheumatology/kem266. [DOI] [PubMed] [Google Scholar]
- 37.Ammitzboll CG, Thiel S, Ellingsen T, Deleuran B, Jorgensen A, Jensenius JC, Stengaard-Pedersen K. Levels of lectin pathway proteins in plasma and synovial fluid of rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2012;32(5):1457–1463. doi: 10.1007/s00296-011-1879-x. [DOI] [PubMed] [Google Scholar]