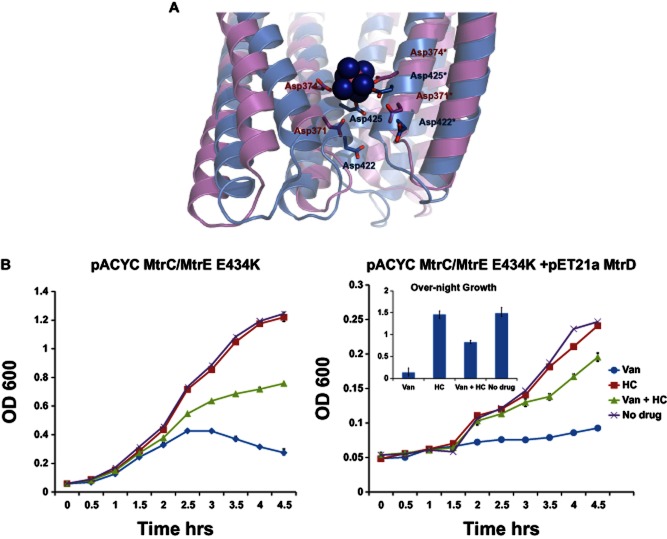
Fig. 2.
Vancomycin entry into cells through MtrE is blocked by hexamminecobalt.
A. A homology model of the MtrE channel based upon a comparative sequence analysis of MtrE and TolC. This model demonstrates that Asp371 and Asp374 in TolC, which form a binding site for hexamminecobalt (III) chloride (HC), are conserved in MtrE and exposed to the internal channel cavity, thus likely forming a similar arrangement to that in TolC. The MtrE model is in blue; two subunits of the TolC–HC complex (1TQQ.pdb) are in magenta; HC is in spacefill; residues Asp371 and Asp374 from TolC, and the homologous Asp422 and Asp425 from MtrE, are shown as sticks.
B. A series of curves showing the relative growth, measured as the OD600, for E. coli cells C43(ΔacrB) transformed with pACYC-MtrC-MtrE(E434K) (left set of graphs) and pACYC-MtrC-MtrE(E434K) and pET21a-MtrD (right set of graphs). Cells were grown in the absence of drugs (×); or in the presence of HC (▪, 25 μM), vancomycin (♦, 6.4 μg ml−1), of HC and vancomycin (▴). Each bar is the average of at least three measurements, with error bars representing the standard deviation of these measurements from the average. Cells transformed with pACYC and pET grew slower than those transformed with pACYC only; but, as shown in the bar chart, attained a comparable OD after overnight growth.