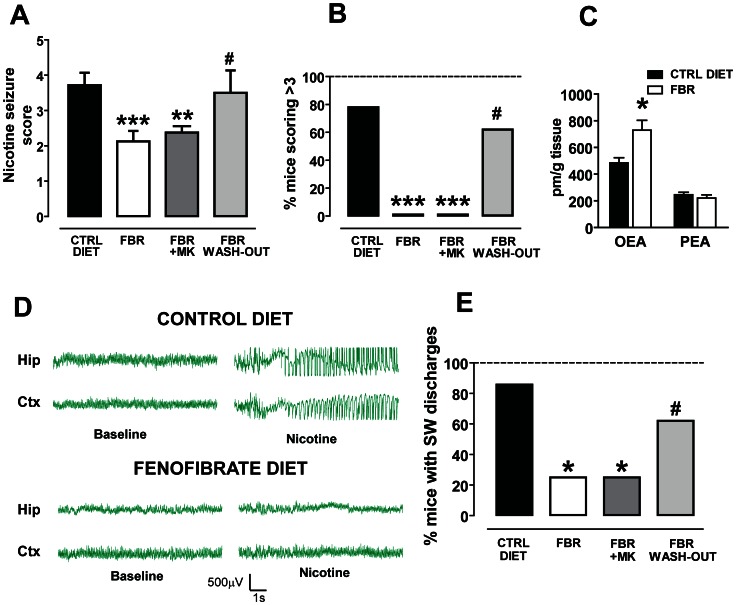
Figure 3. The clinically used PPARα agonist fenofibrate chronically administered with food reduces nicotine-induced seizures and spike-wave activity, and increases the OEA levels in the frontal cortex.
(A) Graph displaying that fenofibrate (n = 8, FBR, 0.3% w/w in the diet for 14 days) reduced the severity of nicotine-induced seizures (nicotine dose: 10 mg/kg, s.c) (***P<0.001 vs. control diet, CTRL DIET, Dunn’s-test). This effect was not abolished by the selective PPARα antagonist MK886 (FBR+MK, 3 mg/kg, i.p.) (n = 8, **P<0.01 vs. CTRL DIET, Dunn’s-test) but by withdrawal of fenofibrate treatment for 14 days (FBR WASH-OUT, n = 8, # P<0.05 vs. FBR and P>0.05 vs. CTRL DIET, Dunn’s test). (B) This graph shows that the percentage of mice undergoing severe nicotine-induced seizures (indexed by scores >3) is significantly attenuated after chronic fenofibrate treatment (***P<0.001 vs. control diet, Fisher’s test) and restored after fenofibrate withdrawal for 14 days (#P>0.05 vs. control diet). MK886 did not reverse this effect (***P<0.001 vs. control diet, Fisher’s test). (C) Chronic activation of PPARα by fenofibrate changes oleoylethanolamide (OEA), but not palmitoylethanolamide, levels within frontal cortex. Concentrations of these endogenous PPARα ligands are expresses as pmol per gram of tissue. Error bars depict S.E.M. (*P<0.05, Student’s t-test). (D) Representative traces of EEG recordings from hippocampal (Hip) and sensorimotor cortical (Ctx) electrodes chronically implanted in mice. In mice fed with control diet 10 mg/kg nicotine elicits bursts of synchronous spike-wave (SW) activity with high-amplitude and low-frequency (most in the delta rhythm range). This activity was suppressed in animals fed with fenofibrate in food pellets. (E) The graph shows the percentage of mice presenting SW discharges following the four treatment protocols. 86% of control diet fed mice (6 out of 7) did show nicotine-induced SW activity. The effects of nicotine were fully blocked in all fenofibrate treated mice, since SW burst were recorded only in none of treated animals (***P<0.01 vs. control diet, Fisher’s test). MK, administered 15 before nicotine, did not restore nicotine-induced SW activity (***P<0.001 vs. control diet, Fisher’s test), whereas fenofibrate washout did (#P>0.05 vs. control diet, Fisher’s test). Data are expressed as mean±95% C.I.