Abstract
Purpose
After a radiological ‘dirty bomb’ incident in a major metropolitan center, substantial numbers of people may be exposed to radiation. However, only a fraction of those individuals will need urgent medical attention. Consequently, a rapid screening test is needed to identify those people who require immediate treatment.
Material and methods
Ten normal human cell lines were screened by enzyme-linked immunosorbent assay (ELISA) for the expression of a dozen secreted cytokines that have been reported to have changes in protein or mRNA levels at 1, 2, and 3 days after 0–10 Gy irradiation using 137Cs gamma rays at 0.82 Gy min−1. After this systematic in vitro screen, we measured changes in the level of a subset of these candidate proteins in plasma from irradiated C57BL/6 mice (n = 3 per group), comparing shams with a single radiation dose (5 Gy X-rays) at 3.7 Gy min−1 at 6 h after irradiation.
Results
We identified four cytokine molecules that had altered levels after radiation exposure, one of which, Interleukin (IL) 6, was consistently elevated after irradiation in vitro and in vivo.
Conclusions
Our findings underscore the potential for IL6 as a marker for an immunoassay-based, rapid, high-throughput biodosimeter.
Keywords: radiation, biodosimeter, immunoassays, ELISA, cytokines, IL6
Introduction
There is an urgent need for a rapid and high-throughput screening tool to identify individuals exposed to radiation in the event of a ‘dirty bomb’ incident. After such a terrorist attack, large numbers of people will need to be tested but only a small proportion of the population will require medical attention (Flynn and Goans 2006). Consequently, the goal of any test designed to detect radiation exposure, a radiation biodosimeter, would be to correctly identify only that minor fraction that has been seriously affected by the radiation dispersal device.
After radiation exposure, the LD50 (dose causing 50% mortality) for healthy adult humans is estimated to be between 3 and 4 Gray (Gy). Individuals exposed to doses below 2 Gy and without other injuries (i.e., burns, etc.) need to be monitored carefully for hematologic damage and treated in response to specific symptoms, but immediate medical attention is not recommended, while doses above 8–9 Gy are usually fatal. Within this window, isolation of patients and antibiotic treatment to prevent infection, blood transfusions and, as a final resort, bone marrow transplants, can raise the LD50 to ~7 Gy (Flynn and Goans 2006; Hall and Giaccia 2006). Consequently, a rapid and accurate test to detect individuals exposed to between 2 and 7 Gy could save a substantial number of lives.
Antibody-based assays are an attractive option for diagnostic tests because they are relatively cheap, non-toxic and yet highly specific. Furthermore, enzyme-linked immunosorbent assay (ELISA) is a test that can be completed in a few hours and is performed in a 96- or 384-well microplate. In addition, ELISA can be fully automated, allowing for high sample throughput while still maintaining the sensitivity required for accurate analysis. As a result, antibody-based assays are among the most commonly chosen techniques for diagnostic tests. The technology underpins a variety of assays that have both commercial and industrial applications as diverse as screening for specific protein expression in plant breeding programs, to detecting viral infections in livestock, to testing in humans for bladder cancer or pregnancy (Armstrong et al. 1984; Evermann and Jackson 1997; Partridge and Hill 2001; Partridge and Appels 2002; Grossman et al. 2005). Given this range of successful applications of antibody-based diagnostics, and the level of technical expertise available, we wanted to assess high-throughput immunoassays, such as ELISA, for developing a radiation biodosimeter.
It is well established that exposure of cells to radiation changes the level and/or activity of a variety of cellular proteins. These include a number of DNA repair enzymes as well as a number of paracrine signaling molecules, such as cytokines, their receptors and downstream cell adhesion molecules (Hallahan and Virudachalam 1997a, 1997b; Amundson et al. 2003, 2004; Smith and Giorgio 2004) Recently, researchers have focused on the changes in the entire proteome using traditional protein detection methods as well as more sophisticated techniques such as Luminex technology. Protein expression profiling, for example for proteins found in blood of exposed individuals, may provide more information about biological response to radiation than gene expression profiling and more accurate dose measurements than could be obtained from measurements of a small number of proteins (Desai et al. 2004; Blakely et al. 2005).
A number of proteins induced by irradiation are either secreted or are expressed on the cell surface. These antigens are an appealing target for high-throughput assays because alterations in extracellular levels may be detected without lysing the cells, and ease of sample preparation is a critical consideration for high-throughput assays. By assaying changes in extracellular protein-level, potentially, blood from a finger prick or cells from a mouth swab could be assayed directly, without time consuming preparative steps which are required, for example, to extract proteins found in the nucleus.
We set out to conduct a systematic in vitro screen of a range of normal human cell lines for the expression of a dozen secreted cytokines that have been reported to have changes in protein or mRNA levels after irradiation. One of the most important, and yet most difficult, aspects of developing a diagnostic assay for humans is effectively validating the technique in a whole organism. Having identified in cultured cells a subset of molecules that have changes in expression or secretion after irradiation, we wanted to perform proof of principle tests on whole organisms by irradiating mice and using ELISA to detect changes in cytokine levels in blood samples, material that would be analogous to samples acquired from humans by a finger prick. The use of samples derived from hematopoietic cells secretions also has other advantages. Due to the mixing that occurs in the circulatory system, samples obtained from individuals with partial body exposure will almost certainly contain cells that have been directly irradiated even if the sample site has not been exposed.
We identified four important cytokine molecules that had altered levels after radiation exposure, one of which, Interleukin (IL) 6, was consistently elevated after irradiation in vitro and in vivo, even at doses as low as 1 Gy. These findings provide a crucial foundation for developing an antibody-based biodosimeter for detecting radiation exposure.
Materials and methods
Cultured cells
The human cell lines used in this study are listed in Table I. BEP2D cells were grown in LHC-8 serum free basal media supplemented with growth factors supplied by the manufacturer (Invitrogen, Carlsbad, CA, USA). HEK, 293T and WI38 cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Biowest, Miami, FL, USA) and L-glutamine (Invitrogen). MRC5 cells were cultured in Minimum Essential Medium (MEM, Invitrogen), supplemented with vitamins, amino acids, sodium pyruvate and 15% FBS. Normal human lung fibroblasts (NHLF) cells were cultured in fibroblast basal medium supplemented with 2% FBS and other growth additives supplied by the manufacturer (Lonza, Basel, Switzerland). The small airway epithelial (SAE) cells were cultured in a serum free saline-adenine-glucose-mannitol (SAGM) medium supplemented with various growth factors supplied by the manufacturer (Lonza). MD human macrophage cells were grown in Iscove’s modified Eagle’s medium (IMEM, Invitrogen) supplemented with 10% FBS, L-glutamine, thymidine, hypoxanthine and 2-mercaptoethanol (Invitrogen). All cultures were grown with antibiotics and were maintained at 37°C in a humidified 5% CO2 incubator. For irradiation, 50K cells were plated In T25 flask and allowed to spread for 24 h. One hour prior to irradiation, the media was changed and then subsequently changed every 24 h and kept for analysis in the ELISA. Cells were exposed to radiation from a GammaCell 40 137Cs irradiator (dose rate, 0.82 Gy min−1) of Columbia University. Transforming growth factor beta (TGFβ content was determined after acid treatment of supernatants to release the molecule fromits latent complexes as described by the ELISA kit manufacturer.
Table I.
Origin and source of cell lines used in this study.
| Cell Line | Tissue/source |
|---|---|
| BEP2D | Normal human bronchial epithelial cells |
| HCF | Human cardiac fibroblasts |
| ScienCell Research Laboratories (San Diego, CA, USA) | |
| HEK | Human embryonic kidney cells |
| ATCC | |
| 293T | HEK containing SV40 Large T antigen |
| ATCC | |
| HUVEC | Human umbilical vein endothelial cells |
| Lonza Group (Switzerland) | |
| MRC5 | Human fetal lung |
| Coriell Cell Repositories (NJ, USA) | |
| NHLF | Normal human lung fibroblasts |
| Lonza Group (Switzerland) | |
| SAE cells | Small airway epithelial cells |
| (Piao, Liu et al. 2005) | |
| WI38 | Human lung fibroblast |
| ATCC | |
| MD | Human macrophage cells |
| ATCC |
Animals
C57BL/6J mice were obtained from Dr Takehiko Nohmi of Division of Genetics and Mutagenesis, National Institute of Health Sciences, Tokyo, Japan. Adult female mice were irradiated with X-rays (X-rays dose rate 3.7 Gy min−1 at 300 KeV). Mice were placed in cylindrical containers with 32 cm diameter and 12 cm height for irradiation and female controls were sham irradiated. Six hours after irradiation, mice were anaesthetised and blood collected from the occipital vein using micro-hematocrit capillary tubes coated with ammonium heparin (Thermo Fisher Scientific, Rockford, IL, USA) after which the animals were sacrificed. Blood was processed immediately by centrifugation (700 g, 15 min, 4°C) to remove platelets. The use of the animals and the experimental protocol were previously approved by the Columbia University Institutional Animal Care and Use Committee. The animals were treated humanely and with regard towards the alleviation of pain and suffering.
Antibodies
Antibodies pairs used in sandwich ELISA for this study was all commercially available. Kits to detect Epidermal growth factor (EGF), Inter-Cellular Adhesion Molecule 1(ICAM1), IL1α, IL1β, IL4, IL6, IL10, TGFβ, Tumour necrosis factor-alpha (TNFα) and Vascular endothelial growth factor (VEGF) were from Invitrogen. Antibody pairs for Fas and Matrix metallopeptidase 8 (MMP8) detection were from R&D Systems (Minneapolis, MN, USA).
ELISA
Microwell plates (eBioscience, San Diego, CA, USA) were coated with 100 ul of capture antibody in coating buffer (Invitrogen) overnight at 4°C. For blocking, 200 ul of 3% bovine serum albumin (BSA, Sigma-Aldrich, St Louis, MO, USA) in phosphate buffered saline (PBS, Invitrogen) was added to wells and incubated for 1 h at 20°C. Plasma was diluted 1:5 in 2% BSA in PBS containing 0.05% Tween 20 (Sigma-Aldrich) (PBST) and cell culture supernatant was added directly (50 and 100 ul, respectively) to the wells followed immediately by addition of biotin-conjugated detection antibody, diluted in 50 ul of 2% BSA-PBST. After 90 min incubation at 20°C with shaking, plates were washed (4 × PBST) and streptavidin-HRP was added, diluted in 100 ul of 2% BSA-PBST. Following 1 h incubation at 20°C, microwells were washed four times and 100 ul of tetramethylbenzidine (TMB, Thermo Scientific) added for 15–30 min at 20°C. Reactions were stopped by addition of 0.9M H2SO4 (50 ul) and product absorbance was determined at 450 nm. Non-specific background (measured at 630 nm) was subtracted. The total assay time was approximately 3 h and 45 min. For luminescence ELISA the procedure was similar except that white microwell plates were used and reactions were detected with a chemiluminescent substrate (Thermo Scientific). Glow luminescence was determined using photomultiplier tube detector (BioTek, Winooski, Vermont, USA). For cell culture supernatant standard were diluted directly in PBST, for mouse plasma standards were diluted in PBST containing 20% FBS.
Results
Screening human cell lines for cytokine expression after irradiation
More than 260 proteins have been reported to be responsive to radiation (Marchetti et al. 2006) and we wanted to perform a systematic examination of the most promising of the candidate antigens. We used a range of cultured human cells, including hematopoietic cells, to detect changes in expression of a variety of secreted proteins and used ELISA as the detection system. Subsequently, we assessed whether the cells increased the expression of these proteins after irradiation, or if cells that were initially negative could be induced to express the protein above the threshold of detection in our assay. We selected human cell lines that were readily available in our laboratory and chose normal cell lines in preference to tumour cell lines. We then chose antigens whose protein expression had been reported to change after exposure to radiation, and in most cases selected proteins for which changes had been reported in multiple independent laboratories (Marchetti et al. 2006). In addition, we limited our testing to antigens for which antibody ELISA kits were commercially available.
The results from this initial screen of cell culture medium from 10 cell lines for expression of a dozen different antigens are given in Table II. Two results were obvious from this preliminary analysis: (i) Most antigens were not expressed in the media from any of the cell lines tested; and (ii) those antigens that were clearly positive in ELISA were usually expressed in multiple cell lines. Of the 12 antigens screened only three gave at least a moderate response in ELISA in multiple cell types; IL6, TGFβ and VEGF. Importantly, both IL6 and TGFβ have been previously identified as promising target antigens for radiation biodosimetry (Marchetti et al. 2006).
Table II.
ELISA detection of cytokines in supernatants from normal human cell cultures.
| Cell line |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antigen | BEPD2 | HCF | HEK | 293T | HUVEC | MRC5 | NHLF | SAEC | WI38 | MD |
| EGF | ++ | − | − | − | − | n.d. | − | ++ | − | n.d. |
| Fas | − | ± | ± | − | − | n.d. | + | ± | ± | n.d. |
| ICAM-1 | − | n.d. | − | − | − | − | − | − | n.d. | n.d. |
| IL1-α | − | − | − | − | − | − | − | − | − | n.d. |
| IL1-β | − | − | − | − | − | − | − | − | − | − |
| IL-4 | n.d. | − | − | − | − | n.d. | − | − | − | n.d. |
| IL-6 | − | +++ | − | − | +++ | ++ | +++ | + | + | + |
| IL-10 | n.d. | − | − | − | − | n.d. | − | − | − | n.d. |
| MMP-8 | n.d. | − | − | − | − | n.d. | − | − | ± | n.d. |
| TGFβ | + | ± | + | + | ++ | + | ++ | ± | + | − |
| TNFα | − | − | − | − | − | − | − | − | − | − |
| VEGF | +++ | + | + | ++ | − | ++ | +++ | ++ | + | n.d. |
ELISA absorbance was determined at 450 nm. Non-specific background (measured at 630 nm) was subtracted. − = zero; ± = <0.1; + = 0.1 – 0.3; ++ = 0.3 – 1.0; +++ = >1.0; n.d. = Not determined. Please see Materials and methods for more details.
In addition to the test for basal expression, we also wanted to determine if any of these cells could be induced to express the antigens after irradiation, even if they were initially negative. All cell lines were exposed to moderate to high levels of γ-radiation (2, 5 or 10 Gy) and the culture media tested for secreted proteins. However, none of the cell lines negative for the antigens tested was positive in ELISA after irradiation (not shown), indicating that the antigens were not expressed de novo after irradiation, at least at the level of detection in colorimetric ELISA.
It is important to note that, for most antigens, the minimum level of detection using chromogenic substrates in a colorimetric ELISA is ~10 pg/ml. However, chemiluminescent substrates are ~10 times more sensitive than colorimetric substrates (Sachdeva and Asthana 2007). Consequently, we rescreened a subset of cell lines (Primary Human Umbilical Vein Endothelial Cell (HUVEC), NHLF, SAE cells, MD) in ELISA using a chemiluminescent substrate for detection. We focussed particularly on those antigens that had a poor response, or were weakly positive, in the colorimetric ELISA, such as Fas, ICAM1, IL1α, IL1β, IL10, MMP8, TGFβ and TNFα (Table II). However, similar to the results obtained with the colorimetric assay, none of the antigens tested were positive in ELISA using chemiluminescent substrates with or without irradiation (not shown).
IL6 expression increases after irradiation
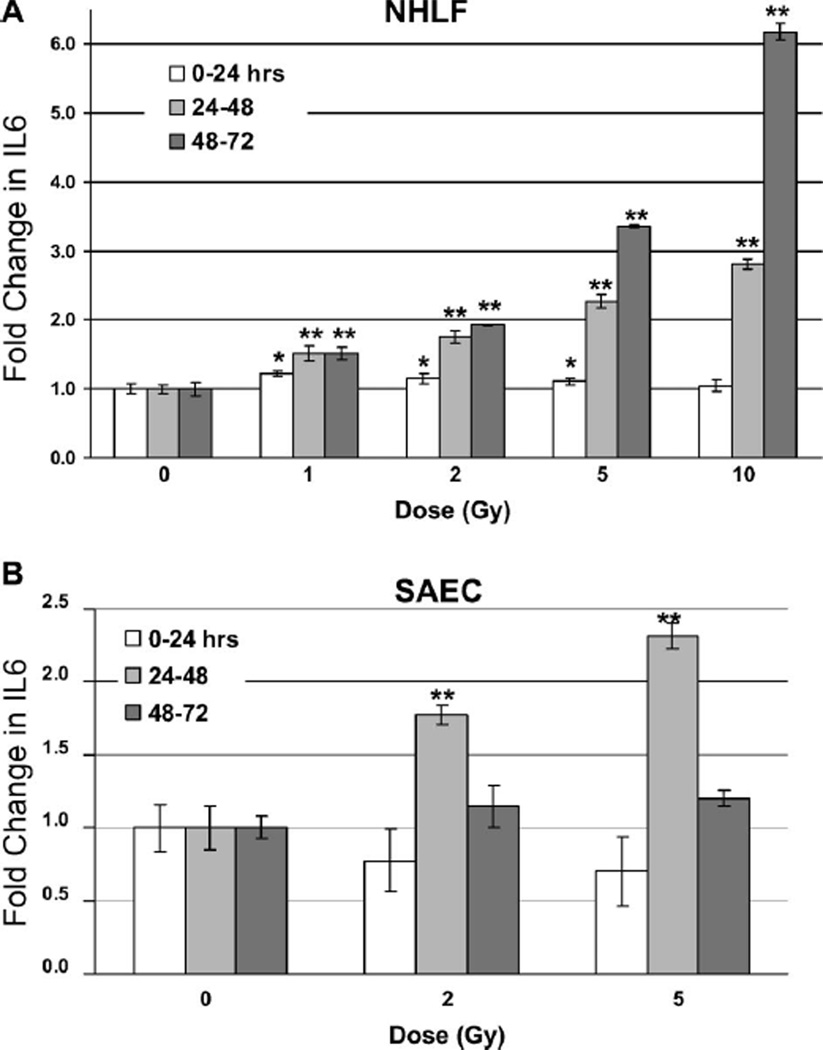
Interestingly, some cell lines that were positive for IL6 (and, to a small degree, Fas – not shown) did display a consistent increase in concentration in the culture media after irradiation. In NHLF cells, there was a small but significant change in IL6 levels 24 h after irradiation, even at doses as low as 1 Gy (0–24 h = 1.2-fold ± 0.03; 24–48 h = 1.5-fold ± 0.1; 48–72 h = 1.5-fold ± 0.09). Importantly, at time points later than 24 h, secreted IL6 in NHLF cultures increased much more dramatically in a time- and dose-dependent manner (Figure 1A). Forty-eight hours after exposure, there was a 2-fold increase (±0.1) in IL6 levels in cultures exposed to 5 Gy, and a more than 3-fold (±0.02) increase after 72 h. In cells exposed to 10 Gy, IL6 levels increased more than 6-fold (1604 pg/ml) compared to controls (260 pg/ml) after 72 h. Even cells exposed to much lower doses displayed an almost 2-fold increase (±0.01) in IL6 detected in the media after irradiation (2 Gy, 72 h, 502 pg/ml) compared to controls. This was important because for most antibody-based diagnostic applications an approximately 2-fold change is necessary for discriminating between samples (Partridge and Hill 2001). Importantly, even at doses as low as 1 Gy, there was a greater than 50% increase in IL6 detected after 48 and 72 h in NHLF cells compared to controls (Figure 1A). It should be noted that although there was a statistically significant difference (P < 0.05) in response between controls and irradiated cultures after 24 h (1, 2 and 5 Gy, Figure 1A), this may not be sufficient for discriminating between samples in the field where there is a greater inherent variability.
Figure 1.
ELISA detection of IL6 in NHLF and SAE cell culture supernatant after irradiation. 50,000 cells were plated in flasks and allowed to spread overnight. The next day the media was replaced and the cultures γ-irradiated with the indicated doses. The media was changed every 24 hours and tested for expression of IL6 by ELISA. Data represent averages of triplicate determinations from two separate assays and are expressed as the fold increase in signal for supernatant from irradiated cell cultures compared to the control cultures for each time point (error bars are the standard deviation from two independent measurements). Statistical comparison between control and sample marked with asterisks (*P < 0.05, **P < 0.01, Student’s t-test). Error bars indicate the standard error of the mean (SEM) for three independent experiments.
In addition to NHLF cells, we also observed an increase in IL6 levels in MD (not shown) and SAE (Figure 1B) cell media after irradiation. When SAE cells were exposed to 5 Gy there was a greater than 2-fold increase (±0.08) in IL6 expression (107 pg/ml) after 48 h compared to controls (46 pg/ml). Even at doses as low as 2 Gy, there was an almost 1.8-fold increase (±0.06) in IL6 detected after 48 h (82 pg/ml). For the 48-h time point, these results were remarkably consistent with radiation-induced levels of IL6 in NHLF cultures. However, at the 72-h time point, the increase in IL6 after irradiation for SAE cell cultures, although clearly measurable, was far less dramatic than for NHLF cells. However, in SAE cells after exposure to high doses (10 Gy), IL6 levels were lower than in cells exposed to 5 Gy after 48 h, and, after 72 h, IL6 levels in cultures exposed to 10 Gy were actually lower than controls (not shown). This is likely due to a higher level of necrosis in SAE cells exposed to 10 Gy and suggests a greater sensitivity of these cells to radiation.
In vivo model for detecting radiation-induced changes in cytokine levels
Having identified a consistent increase in IL6 in numerous cell lines after radiation exposure, we wanted to determine whether this candidate biomarker was elevated in whole organisms, and not simply cultured cells. Furthermore, we wanted to establish that the protein could be detected in materials that could, in humans, be obtained using minimally invasive techniques, such as a blood from finger prick.
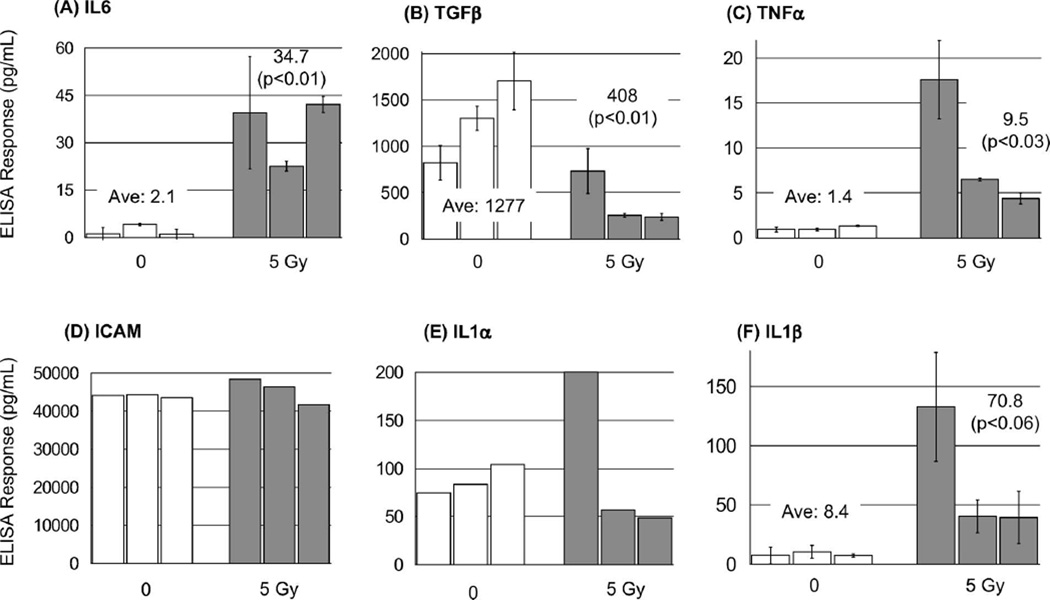
To accomplish this, three female mice were irradiated with 5 Gy X-rays and, 6 h later, blood was obtained and plasma analysed from these mice for an increase in IL6 levels. The relative biological effectiveness (RBE) of ionising irradiation varies with linear energy transfer (LET). Because X-rays and γ-rays have similar LET, the RBE of these two types of irradiation is similar. The results from these experiments clearly showed that IL6 levels in each of the three irradiated mice were all higher than the levels in the highest control animal (Figure 2A). In fact, there was a 40-fold increase in IL6 between the irradiated animal with the highest level and the control mouse with lowest IL6 plasma content. Collectively, the average IL6 levels were 16-fold greater (±5.4) in the plasma of irradiated mice compared to controls (P < 0.01, Student’s t-test).
Figure 2.
ELISA detection of IL6, TGFβ, TNFα, ICAM, IL1α and IL1β in plasma from irradiated mice. Groups of three female mice were sham irradiated or exposed to 5 Gy X-rays and plasma obtained after 6 hours and tested in ELISA for changes in cytokine levels. Data are averages of triplicate determinations from one or two independent measurements. Error bars are the standard deviation from two independent measurements. Averages for each group (given with P value on the chart above each group) and statistical comparison is derived from all 18 measurements for each, control vs. irradiated (Student’s t-test). Error bars indicate the standard error of the mean (SEM) for three independent experiments.
This quite dramatic result prompted us to rescreen some of the antigens that showed modest (but not statistically significant) changes in level after irradiation of cultured cells, or those were prominent cytokines important in cell signaling or tumourigenesis. Using plasma from irradiated mice we tested for changes in the levels of five other cytokines, IL1α, IL1β, TNFα, TGFβ and ICAM (Figure 2B, C, D, E and F). Interestingly, after irradiation plasma levels of three of these cytokines (IL1β, TNFα, TGFβ) showed substantial changes compared to controls (+8.4-fold ± 3.8; +6.7-fold ± 2.9; −3.1-fold ± 0.47, respectively – although IL1β just escaped statistical significance) (Figure 2B, C, and F).
Discussion
Hundreds of proteins have been reported to be responsive to radiation (Marchetti et al. 2006), ostensibly providing a large pool of potential candidates for a protein-based radiation biodosimeter. A number of groups have investigated cytokine response to radiation in animal models (Rubin et al. 1992; Barcellos-Hoff et al. 1994; Hallahan and Virudachalam 1997a; Hong et al. 1999; Fedorocko et al. 2002; Lynch et al. 2003). However, these observations were made with tissues that were acquired using an invasive biopsy. This would not be suitable for a high-throughput screen in a human population. Here, we used immunoassays as the detection method because they are well suited to high-throughput diagnostic applications. We identified several potential antigens, particularly IL6, which had changes in expression after irradiation in cultured cells for further analysis in samples from animals exposed to radiation. The changes in IL6 level after irradiation in our murine studies were comparable to findings from non-human primates. Monkeys exposed to 6 Gy X-rays had increased IL6 plasma levels of more than 13-fold after 24 h, a figure similar to the ~16-fold increase in IL6 levels we observed 6 h after 5 Gy X-irradiation in mice (Ossetrova et al. 2007).
The response to radiation of the major cytokines examined here has been previously studied in other contexts. IL6 is a pro-inflammatory cytokine that is a critical regulator of the acute phase response to trauma, including radiation exposure (Choy and Panayi 2001; Dent et al. 2003; Heinrich et al. 2003). IL6 has been identified as a marker for radiation exposure in laboratory studies using cancer cells exposed to X-rays (Beetz et al. 1997), and fibroblasts and mouse lung tissue exposed to γ-irradiation (Brach et al. 1993; Fedorocko et al. 2002). These studies have detected increases in both protein expression and mRNA level after exposure to radiation from different sources. The evidence of increased mRNA levels after radiation exposure suggests that the elevated IL6 levels detected in this study may have been due to increased protein expression, rather than greater secretion into the media of previously synthesised protein. Interestingly, IL6 levels were also increased in survivors of the atomic bomb in Hiroshima City decades after exposure (Hayashi et al. 2003). However, it is not known what the level of IL6 was in individuals in the days and weeks after the bomb was detonated.
TGFβ is involved in regulating a range of cellular processes including differentiation, proliferation and apoptosis (Shi and Massague 2003). After irradiation, the level of latent TGFβ has been shown to decrease in tissues from rodents (Barcellos-Hoff et al. 1994) which is consistent with our findings that total TGFβ plasma levels decreased after irradiation, and with the anti-inflammatory role of TGFβ (Blobe et al. 2000), a function that could inhibit normal inflammation after irradiation. Finally, studies on mice have identified increases in IL1β protein in brain tissue and increased mRNA in the lung (Hong et al. 1999; Lynch et al. 2003).
As mentioned, there are a number of potential biomarkers of radiation exposure. In fact, excellent radiation-specific methodologies for accurate dose assessment, such as the measurement of dicentric chromosomes, have existed for decades (International Atomic Energy Agency 2001). However, these techniques are not well suited for use in the event of radiological terrorist incident because they are neither rapid (> 48 h to begin analysis) nor high-throughput (scores of samples per experimenter/day), and the analysis requires well-trained personnel (Lloyd et al. 2000; Léonard et al. 2005). In contrast, immunoassays take only a few hours from sampling to final results, can be fully automated to process tens of thousands of samples at a time and provides simple numerical readouts that require little technical analysis. It is likely that multiple biomarkers will be needed to unambiguously identify any exposed individuals and the development of a complete proteome profile for irradiated cells will be important in developing an effective biodosimeter (Ivey et al. 2009). Potentially, a suite of antibodies labelled with different fluorophores could simultaneously detect different antigens in the same assay, likely improving the level of sensitivity.
It is important to note that there can be a large variability in response between two individuals in a population with a heterogeneous genetic background. In fact, our results indicate that the plasma cytokine levels reported here in some cases differ between individuals within an exposure group by more than 4-fold (Figure 2E). This is not dissimilar to the degree of variability in micronucleus incidence (also a potential radiation biomarker) observed in a population even when the gender and age group of the individuals remain the same (Fenech 1998). Furthermore, both mRNA expression levels and plasma protein levels of a number of radio-responsive genes have been shown to differ between individuals by an order of magnitude after irradiation (Correa and Cheung 2004; Grace and Blakely 2007; Ossetrova et al. 2007).
In this study we wanted employ immunoassays to systematically investigate the change in cytokine levels in a range of normal cell lines after exposure to radiation and use the findings as a guide for detecting changes in cytokine levels from irradiated whole animals. Our findings underscore the potential for IL6 as a marker for an immunoassay-based, rapid, high-throughput biodosimeter. The next stage of this work will be to assess the ability of IL6 (and other proteins) to serve as a radiation biomarker at different time points after exposure to different doses of irradiations. It will then be critical to confirm efficacy of these assays in larger populations.
Acknowledgements
This publication was supported by National Institutes of Health grant CA 49062, and by grant U19 AI067773, the Center for High-Throughput Minimally Invasive Radiation Biodosimetry, from the National Institutes of Health/National Institute of Allergy and Infectious Diseases.
Footnotes
Declaration of interest: The authors have no conflicts of interest. They alone are responsible for the content and writing of the paper.
References
- Amundson SA, Grace MB, McLeland CB, Epperly MW, Yeager A, Zhan Q, Greenberger JS, Fornace AJ., Jr Human in vivo radiation-induced biomarkers: Gene expression changes in radiotherapy patients. Cancer Research. 2004;64:6368–6371. doi: 10.1158/0008-5472.CAN-04-1883. [DOI] [PubMed] [Google Scholar]
- Amundson SA, Lee RA, Koch-Paiz CA, Bittner ML, Meltzer P, Trent JM, Fornace AJ., Jr Differential responses of stress genes to low dose-rate gamma irradiation. Molecular Cancer Research. 2003;1:445–452. [PubMed] [Google Scholar]
- Armstrong EG, Ehrlich PH, Birken S, Schlatterer JP, Siris E, Hembree WC, Canfield RE. Use of a highly sensitive and specific immunoradiometric assay for detection of human chorionic gonadotropin in urine of normal, nonpregnant, and pregnant individuals. The Journal of Clinical Endocrinology and Metabolism. 1984;59:867–874. doi: 10.1210/jcem-59-5-867. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. The Journal of Clinical Investigation. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetz A, Messer G, Oppel T, van Beuningen D, Peter RU, Kind P. Induction of interleukin 6 by ionizing radiation in a human epithelial cell line: Control by corticosteroids. International Journal of Radiation Biology. 1997;72:33–43. doi: 10.1080/095530097143518. [DOI] [PubMed] [Google Scholar]
- Blakely WF, Salter CA, Prasanna PG. Early-response biological dosimetry – recommended countermeasure enhancements for mass-casualty radiological incidents and terrorism. Health Physics. 2005;89:494–504. doi: 10.1097/01.hp.0000175913.36594.a4. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. The New England Journal of Medicine. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Brach MA, Gruss HJ, Kaisho T, Asano Y, Hirano T, Herrmann F. Ionizing radiation induces expression of interleukin 6 by human fibroblasts involving activation of nuclear factor-kappa B. The Journal of Biological Chemistry. 1993;268:8466–8472. [PubMed] [Google Scholar]
- Correa CR, Cheung VG. Genetic variation in radiation-induced expression phenotypes. American Journal of Human Genetics. 2004;75:885–890. doi: 10.1086/425221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. The New England Journal of Medicine. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiation Research. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Desai N, Wu H, George K, Gonda SR, Cucinotta FA. Simultaneous measurement of multiple radiation-induced protein expression profiles using the Luminex (TM) system. Advances in space research: The official Journal of the Committee on Space Research (COSPAR) 2004;34:1362–1367. doi: 10.1016/j.asr.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Evermann JF, Jackson MK. Laboratory diagnostic tests for retroviral infections in dairy and beef cattle. The Veterinary Clinics of North America. Food Animal Practice. 1997;13:87–106. doi: 10.1016/s0749-0720(15)30366-2. [DOI] [PubMed] [Google Scholar]
- Fedorocko P, Egyed A, Vacek A. Irradiation induces increased production of haemopoietic and proinflammatory cytokines in the mouse lung. International Journal of Radiation Biology. 2002;78:305–313. doi: 10.1080/09553000110104614. [DOI] [PubMed] [Google Scholar]
- Flynn DF, Goans RE. Nuclear terrorism: Triage and medical management of radiation and combined-injury casualties. The Surgical Clinics of North America. 2006;86:601–636. doi: 10.1016/j.suc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Fenech M. Important variables that influence base-line micronucleus frequency in cytokinesis-blocled lymphocytes-a biomarker for DNA damage in human populations. Mutation Research. 1998;404:155–165. doi: 10.1016/s0027-5107(98)00109-2. [DOI] [PubMed] [Google Scholar]
- Grace MB, Blakely WF. Transcription of five p53- and Stat-3-Inducible genes after ionizing radiation. Radiation Measurements. 2007;42:1147–1151. [Google Scholar]
- Grossman HB, Messing E, Soloway M, Tomera K, Katz G, Berger Y, Shen Y. Detection of bladder cancer using a point-of-care proteomic assay. The Journal of the American Medical Association. 2005;293:810–816. doi: 10.1001/jama.293.7.810. [DOI] [PubMed] [Google Scholar]
- Hall EJ, Giaccia AJ. Radiobiology for the radiologist. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Hallahan DE, Virudachalam S. Intercellular adhesion molecule 1 knockout abrogates radiation induced pulmonary inflammation. Proceedings of the National Academy of Sciences of the USA. 1997a;94:6432–6437. doi: 10.1073/pnas.94.12.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan DE, Virudachalam S. Ionizing radiation mediates expression of cell adhesion molecules in distinct histological patterns within the lung. Cancer Research. 1997b;57:2096–2099. [PubMed] [Google Scholar]
- Hayashi T, Kusunoki Y, Hakoda M, Morishita Y, Kubo Y, Maki M, Kasagi F, Kodama K, Macphee DG, Kyoizumi S. Radiation dose-dependent increases in inflammatory response markers in A-bomb survivors. International Journal of Radiation Biology. 2003;79:129–136. [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)- 6-type cytokine signalling and its regulation. The Biochemical Journal. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. International Journal of Radiation Biology. 1999;75:1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- International Atomic Energy Agency. A manual. Vienna: International Atomic Energy Agency; 2001. Cytogenetic analysis for radiation dose assessment; p. 127. [Google Scholar]
- Ivey RG, Subramanian O, Lorentzen TD, Paulovich AG. Antibody-based screen for ionizing radiation-dependent changes in the Mammalian proteome for use in biodosimetry. Radiation Research. 2009;171:549–561. doi: 10.1667/RR1638.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard A, Rueff J, Gerber GB, Léonard ED. Usefulness and limits of biological dosimetry based on cytogenetic methods. Radiation Protection Dosimetry. 2005;115:448–454. doi: 10.1093/rpd/nci061. [DOI] [PubMed] [Google Scholar]
- Lloyd DC, Edwards AA, Moquet JE, Guerrero-Carbajal YC. Applied radiation and isotopes: Including data, instrumentation and methods for use in agriculture, industry and medicine. 2000;52:1107–1112. doi: 10.1016/s0969-8043(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Moore M, Craig S, Lonergan PE, Martin DS, Lynch MA. Analysis of interleukin-1 beta-induced cell signaling activation in rat hippocampus following exposure to gamma irradiation. Protective effect of eicosapentaenoic acid. The Journal of Biological Chemistry. 2003;278:51075–51084. doi: 10.1074/jbc.M307970200. [DOI] [PubMed] [Google Scholar]
- Marchetti F, Coleman MA, Jones IM, Wyrobek AJ. Candidate protein biodosimeters of human exposure to ionizing radiation. International Journal of Radiation Biology. 2006;82:605–639. doi: 10.1080/09553000600930103. [DOI] [PubMed] [Google Scholar]
- Ossetrova NI, Farese AM, MacVittie TJ, Manglapus GL, Blakely WF. The use of discriminant analysis for evaluation of early-response multiple biomarkers of radiation exposure using non-human primate 6-Gy whole-body radiation model. Radiation Measurements. 2007;42:1158–1163. [Google Scholar]
- Partridge MAK, Appels R. Simple ELISA detection of a new polymorphic Ha locus encoded protein. Journal of Cereal Science. 2002;35:189–200. [Google Scholar]
- Partridge MAK, Hill AS. Two-site sandwich ELISA for discriminating different Gli-1 (gliadin)/Glu-3 (LMW-glutenin subunit) alleles in hexaploid wheat. Cereal Chemistry. 2001;78(3):294–302. [Google Scholar]
- Rubin P, Finkelstein J, Shapiro D. Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: Interrelationship between the alveolar macrophage and the septal fibroblast. International Journal of Radiation Oncology, Biology, Physics. 1992;24(1):93–101. doi: 10.1016/0360-3016(92)91027-k. [DOI] [PubMed] [Google Scholar]
- Sachdeva N, Asthana D. Cytokine quantitation: Technologies and applications. Frontiers in Bioscience. 2007;12:4682–4695. doi: 10.2741/2418. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Smith RA, Giorgio TD. Quantitation and kinetics of CD51 surface receptor expression: Implications for targeted delivery. Annals of Biomedical Engineering. 2004;32(5):635–644. doi: 10.1023/b:abme.0000030230.81832.99. [DOI] [PubMed] [Google Scholar]