Figure 8.
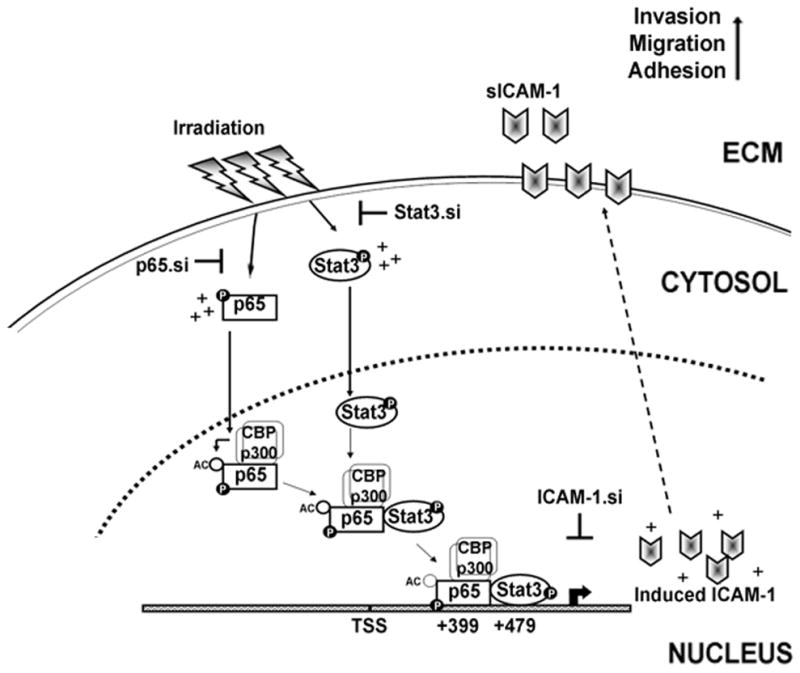
Schematic representation of direct nuclear interaction and reciprocal regulation of NF-κB (p65) and Stat3 in glioma. IR treatment increased phosphorylation of both p65 and Stat3 which eventually translocate into the nucleus. In the nucleus, CBP/p300 acetyl transferases bind to the p-p65, transfer acetyl group and acetylate p65. The p-Stat3 directly interacts with p-p65 at transactivation domain and forms p-p65/p-Stat3 complex in the nucleus. IR elevates the p-p65/p-Stat3 complex formation and subsequent binding to the adjacent NF-κB (+399) and Stat3 (+479) consensus sequences in the ICAM-1 intron-1. As a result, there is a significant increase in ICAM-1 transcription, membrane localization and an increase in sICAM-1 levels in the extracellular milieu of IR-treated cells. This mechanism leads to enhanced adhesion, invasion and migratory properties in post-irradiated cells. On the other hand, the p65/Stat3 interactions were abrogated by specific siRNA-mediated knockdown of p65 and Stat3. IR-induced ICAM-1 upregulation, cell invasion and migration were abrogated by using ICAM-1.siRNA. In summary, our results demonstrated that the elevated functional association of p65 and Stat3 as a function of IR in enhancing glioma malignancy through ICAM-1 upregulation. Hence, blockade of p65 and Stat3 binding provides a potential therapeutic approach in the future treatment of glioma.
