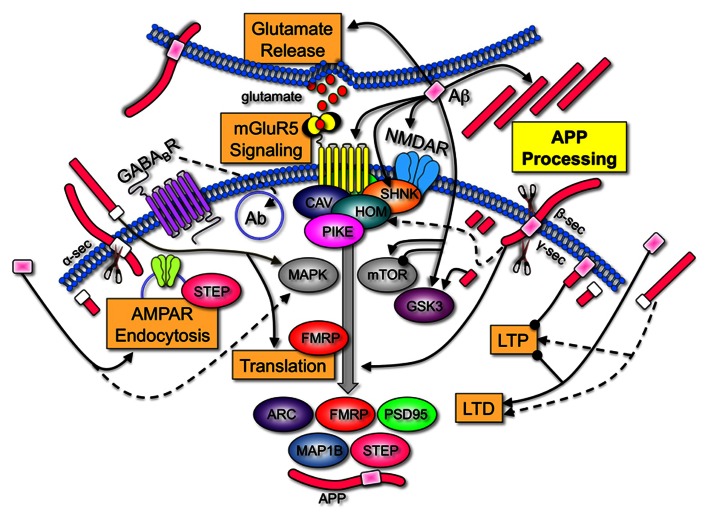
FIGURE 1.
Amyloid-beta protein precursor and Aβ are key regulators of synaptic activity. APP is processed by α-, β-, and/or γ-secretases to produce soluble N-terminal domains of APP (sAPPα and sAPPβ), Aβ and C-terminal fragments. Aβ increases LTD, inhibits LTP (Koffie et al., 2011), induces calcium-dependent synaptic vesicle depletion at the presynaptic membrane (Parodi et al., 2010), binds to numerous postsynaptic surface proteins including NMDAR (Danysz and Parsons, 2012), activates mGluR5 signaling (Casley et al., 2009; Renner et al., 2010), induces Arc and APP expression (Lacor et al., 2004; Westmark et al., 2011b) and interferes with normal NMDAR and 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid receptor (AMPAR) trafficking by triggering receptor internalization (Hsieh et al., 2006; Ma and Klann, 2012). Group 1 mGluRs are anchored to NMDAR via a chain of scaffolding proteins including the long isoforms of Homer, Shank, and postsynaptic density protein 95 (PSD95). Aβ induces disassembly of Shank1 and Homer1b clusters (Roselli et al., 2009). Group 1 mGluRs are upstream of two pivotal signaling pathways, PI3 kinase/Akt/mTOR (mammalian target of rapamycin)/p70S6K and Ras/MAPK (mitogen-activated protein kinase)/p90S6K. Both of these pathways regulate the phosphorylation status of eIF4E, 4E-BPs, and ribosomal protein S6, which positively influence protein synthesis. Aβ and sAPP activate MAPKERK (Young et al., 2009; Chasseigneaux et al., 2011). Aβ both inhibits and activates mTOR signaling (Ma et al., 2010; Caccamo et al., 2011), and activates GSK3β (Takashima et al., 1996), a key regulator of numerous signaling pathways. APP, sAPP, and the intracellular C-terminal fragments also affect synaptic homeostasis. APP anchors cytoplasmic polyadenylation element binding factor (CPEB) to membranes and promotes polyadenylation-induced translation (Cao et al., 2005). sAPPα increases de novo protein synthesis (Claasen et al., 2009), enhances LTP (Taylor et al., 2008), shifts the frequency-dependency for induction of LTD (Ishida et al., 1997), and disrupts APP dimers at the plasma membrane (Gralle et al., 2009). While there is only a 17-amino acid difference between the differentially processed N-terminal fragments, sAPPα possesses synaptotrophic and neuroprotective activities while sAPPβ can be toxic (Zheng and Koo, 2011). The C-terminal fragment generated after amyloidogenic processing of APP is also neurotoxic and activates GSK3 (Ryan and Pimplikar, 2005). The 104 amino acid C-terminal fragment containing Aβ impairs LTP (Nalbantoglu et al., 1997). The levels of many synaptic proteins corresponding to a number of FMRP target mRNAs are constitutively elevated in the Fmr1KO mouse. A few examples that are regulated by mGluR5 are illustrated [ARC (activity-regulated cytoskeleton-associated protein), FMRP, PSD95, MAP1B (microtubule-associated protein 1B), striatal-enriched protein tyrosine phosphatase (STEP), and APP; Bassell and Warren, 2008; Goebel-Goody et al., 2012]. Overall, these data strongly suggest that APP and Aβ are among key LTD proteins whose over-expression during development play an important role in FXS pathogenesis. Key: major cellular processes (glutamate release, mGluR5 signaling, AMPAR endocytosis, translation, LTD, LTP, and APP processing) are boxed. Receptors (mGluR5, NMDAR, and GABABR) are inserted into the membrane and differentially colored. Scaffolding (Shank, Homer, Caveolin, and Pike), signaling molecules (MAPK, mToR, and GSK3), and translated proteins (ARC, FMRP, PSD95, and MAP1B) are oval-shaped and differentially colored. APP is colored fuchsia with the Aβ portion in light pink for amyloidogenic processing (β- and γ-secretase cleavage) and white for non-amyloidogenic processing (α-secretase cleavage). Secretases are denoted by the scissors symbols. Sharp arrowheads denote activation of a protein or process and rounded arrowheads denote inhibition of a protein or process.