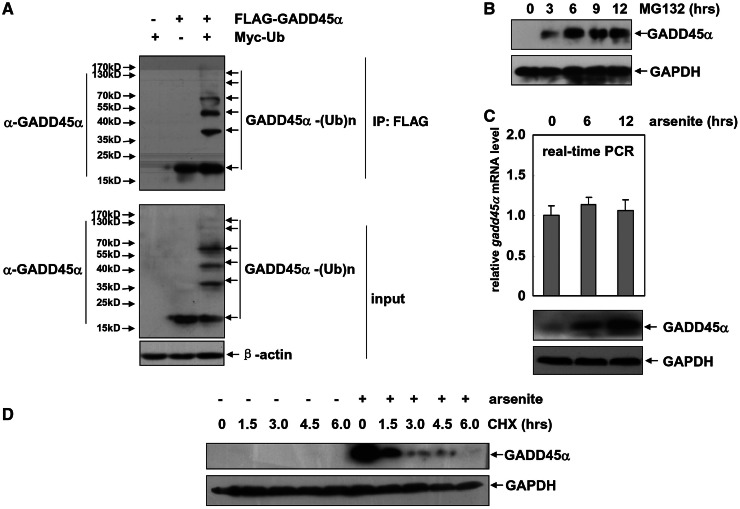
Figure 1.
GADD45α is a liable protein, and its proteasome-dependent degradation is blocked by arsenite in the HepG2 cells. (A) HepG2 cells were transfected with combination of the expression plasmids encoding FLAG–GADD45α or Myc–Ub as indicated. Cell extracts were immunoprecipitated with anti-FLAG antibody, and the ubiquitination of GADD45α was detected with anti-GADD45α antibody. (B) HepG2 cells were treated with MG132 (10 µM) for the indicated time, and then the levels of GADD45α were detected. (C) HepG2 cells were treated with arsenite (20 µM) for the indicated time, and then the induction of gadd45α mRNA and protein levels was detected by real-time PCR or western-blot assays, respectively. (D) HepG2 cells were left untreated or treated with arsenite (20 µM) for 12 h and then subjected to CHX (20 µM) exposure at the indicated time after arsenite withdrawal. The degradation of GADD45α was detected by western-blot assay.