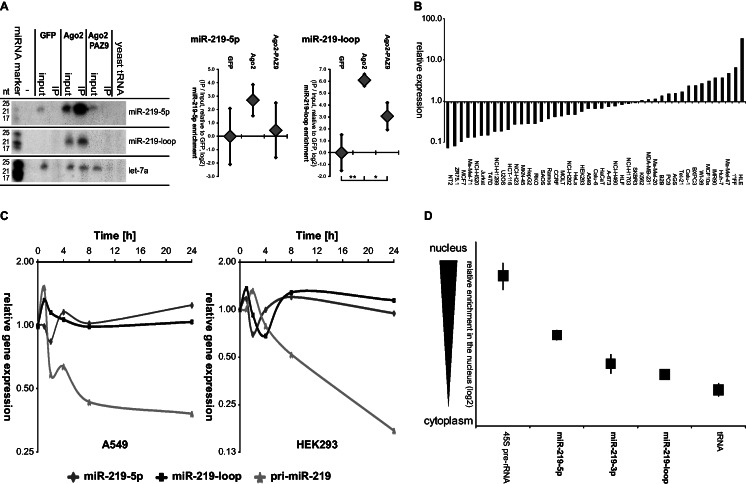
Figure 4.
miR-219-2-loop binds to Ago proteins and is stably expressed in the cytoplasm of a cell. (A) HEK293 cells were transiently co-transfected with miR-219-2 and FLAG-tagged GFP, Ago2 or the binding-defective mutant Ago2-PAZ9. Ago2-binding of the miRNAs was verified by co-immunoprecipitation (IP) using anti-FLAG agarose beads. miRNA expression was subsequently detected by northern blot analysis. Exposure times of the blots: miR-219-loop = 1 day; miR-219-5 p and let-7a = 2 days. The miRNA let-7a served as endogenous control. The input depicts 10% of the IP lysate volume. To quantify the enrichment in the IP, input and IP were subjected to qRT–PCR. Shown is the average enrichment of IP/input normalized to the negative control GFP in five experiments (±SEM). (B) Endogenous mature 5p and loop-miR expression was determined in 45 different cell lines by qRT–PCR analysis. Displayed is the ratio of miR-219-loop to miR-219-5p guide strand expression normalized for the qPCR primer efficiency. (C) miRNA expression in HEK293 and A549 cells treated with the transcription inhibitor actinomycin D was determined by qRT–PCR analysis at the indicated time points. Depicted is the mean expression of three independent experiments as compared with the appropriate DMSO-treated cells. (D) HEK293 cells were fractionated and nuclear and cytoplasmic RNA was isolated. Endogenous pri-miRNA and mature 5p, 3p and loop-miR expression of miR-219-2 was determined by qRT–PCR analysis. The 45S pre-rRNA expression served as nuclear marker, whereas tRNA was used as a cytoplasmic marker. Depicted is the mean of three independent experiments (±SEM) of the ratio between expressions in the nuclear/cytoplasmic fraction in log2 scale (Supplementary Table S5).