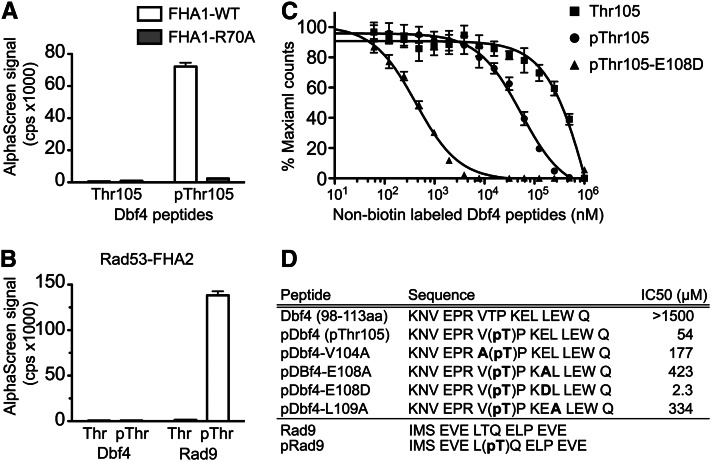
Figure 3.
The Rad53 FHA1 domain directly bound to a T105 phosphorylated Dbf4 peptide. (A) Biotinylated Dbf4 peptides (residues 98–113) were tested for interaction with the purified 6His-FHA1 domain using the AlphaScreen Assay. Data represent the average of three independent experiments ± SEM. (B) Purified 6His-FHA2 domain does not interact with the pThr105 Dbf4 peptide, but does selectively bind a Rad9-phosphorylated peptide. (C) The Dbf4–FHA1 domain interaction was competed by nonbiotinylated, T105-phosphorylated Dbf4 peptide (pThr105), a peptide containing the optimal FHA1-binding sequence (pT105-E108D), but not by the T105 (nonphosphorylated) Dbf4 peptide. (D) Summary of peptide sequences and the IC50 values determined by competition assays.