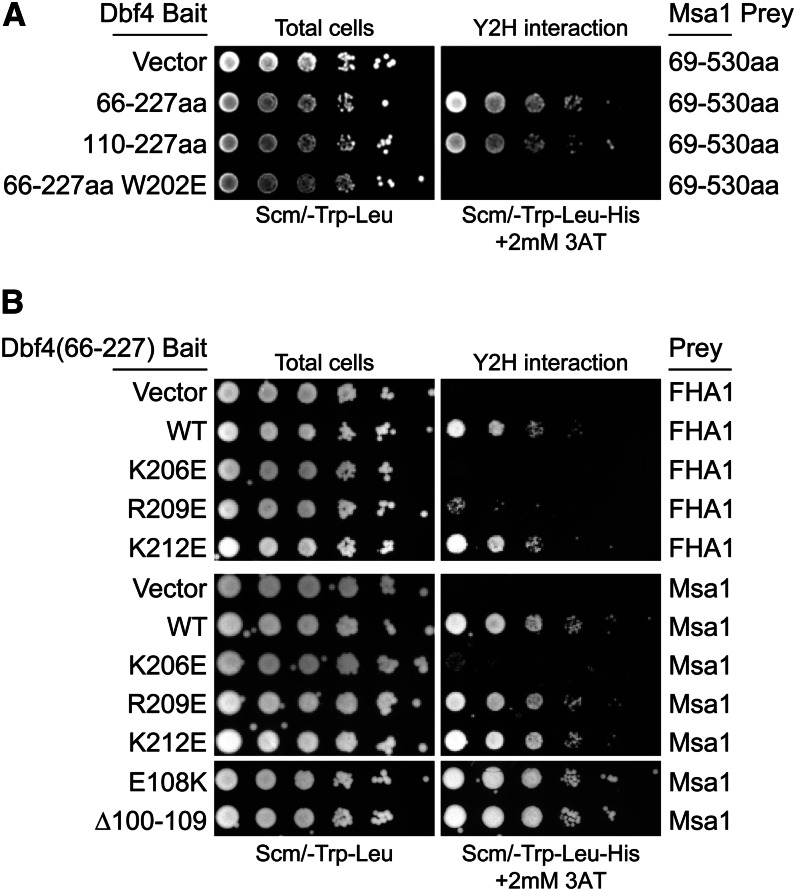
Figure 6.
Both Msa1 and Rad53 interacted with the Dbf4 BRCT domain, but the Rad53 interaction was specifically disrupted by an R209E mutation. (A) Two-hybrid assays with indicated Dbf4 bait and Msa1 prey vectors, spotted as in Figure 1. Msa1 interacts with the BRCT domain alone (Dbf4-110-227). (B) Charge reversal mutations of lysine and arginine residues in the BRCT α3 helix (Matthews et al. 2012) identify R209E as specifically affecting the Rad53 interaction. Dbf4 residues 100–109 are also uniquely required for the Rad53 interaction (Fig. S6C) but not for the Msa1 interaction.