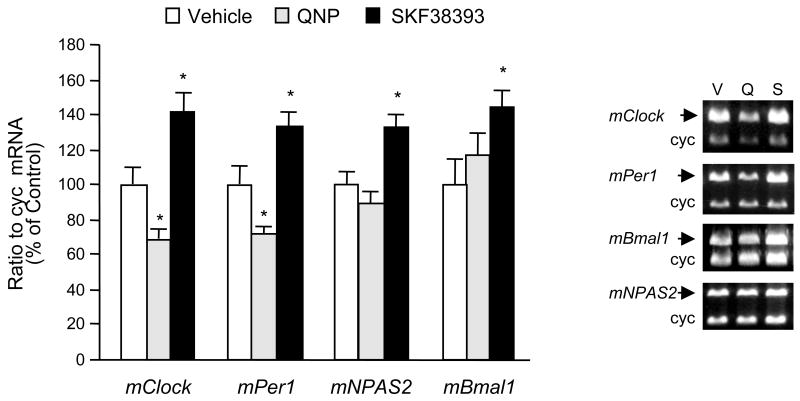
Fig. 2.
The differential regulatory effect of dopamine receptor agonists on clock gene expression in striatal neurons. Quinpirole (1μM) or SKF38393 (1 μM) were applied to the striatal neurons in culture (9 DIV) for 6 h. After treatment, cells were harvested in TRIzol® reagent for mRNA extraction. Specific primers for each clock gene measured were co-amplified with cyclophilin (cyc) primers (see Table 1 for primer sequences) using RT-PCR to quantify mRNA levels for each gene. Radioactivity counts of RT-PCR products (mean ± SEM; n=4 to 6) were calculated as the ratio of each clock gene to cyclophilin and expressed as the ratio of control. *p < .05 in comparison to control group (one-way ANOVA followed by the Dunnett’s multiple comparison test). Quinpirole decreased mRNA levels for both mClock and mPer1 but did not affect mNPAS2 and mBmal1 mRNA levels. On the other hand, SKF38393 increased mRNA levels for all clock genes studied. DMSO was used as the vehicle in control groups in a final concentration of 0.1%. The sample gel electrophoresis pictures in the right panel demonstrate the co-amplification of clock genes and cyclophilin for quantitation assays after the vehicle (V) and dopamine agonist (quinpirole, Q and SKF38393, S) treatments in striatal neurons. The sizes for the individual products are: 534 bp for mClock, 387 bp for mPer1, 471 bp for mBmal1, 458 bp for mNPAS2, and 298 bp for cyc. Please note the drug-induced changes in the density of clock genes except the housekeeping gene cyc.