Abstract
In the tiger salamander retina, visual signals are transmitted to the inner retina via six morphologically distinct types of photoreceptors: large/small rods, large/small single cones, and double cones composed of principal and accessory members. The objective of this study was to determine the morphology of these photoreceptors and their synaptic interconnection with bipolar cells and horizontal cells in the outer plexiform layer (OPL). Here we showed that glutamate antibodies labeled all photoreceptors and recoverin antibodies strongly labeled all cones and weakly labeled all rods. Antibodies against calbindin selectively stained accessory members of double cones. Antibodies against S-cone opsin stained small rods, a subpopulation of small single cones, and the outer segments of accessory double cones and a subtype of unidentified single cones. On average, large rods and small S-cone opsin positive rods accounted for 98.6% and 1.4% of all rods, respectively. Large/small cones, principle/accessory double cones, S-cone opsin positive small single cones, and S-cone opsin positive unidentified single cones accounted for about 66.9%, 23%, 4.5%, and 5.6% of the total cones, respectively. Moreover, the differential connection between rods/cones and bipolar/horizontal cells and the wide distribution of AMPA receptor subunits GluR2/3 and GluR4 at the rod/cone synapses were observed. These results provide anatomical evidence for the physiological findings that bipolar/horizontal cells in the salamander retina are driven by rod/cone inputs of different weights, and that AMPA receptors play an important role in glutamatergic neurotransmission at the first visual synapses. The different photoreceptors selectively contacting bipolar and horizontal cells support the idea that visual signals may be conveyed to the inner retina by different functional pathways in the outer retina.
Keywords: Retinal rod, Cone, Blue cone, Double cones
1. Introduction
In the vertebrate retina, rod and cone photoreceptors are the primary sensory neurons that transduce light energy into electrical signals. Rods are specialized for mediating night vision and function as single-photon detectors, whereas cones are responsible for daylight vision with high temporal resolution but are much less sensitive than rods (Dowling, 1987). Among lower vertebrates, the physiological function and circuitry of rods and cones have been extensively studied in the salamander retina using intracellular and patch clamp recordings (Attwell & Wilson, 1980; Attwell, Wilson, & Wu, 1984; Wu, Gao, & Maple, 2000). It has been shown that rods primarily make synaptic contacts with rod-dominant bipolar and horizontal cells, and cones predominantly make synaptic contacts with cone-dominant bipolar and horizontal cells (Yang & Wu, 1996; Yang & Wu, 1997; Zhang, Zhang, & Wu, 2006). This physiological wiring diagram, commonly referred as parallel processing, ensures visual signals selectively target second order neurons in the inner retina (review see Wassle (2004)). Since there are more than one type of rod and cone in the salamander retina (Mariani, 1986), it is possible that different subtypes of rods and cones selectively contact different types of bipolar and horizontal cells or vice versa. This idea is supported by several studies demonstrating the cone-selective retinal circuitry in the lower vertebrate retinas (Haverkamp, Mockel, & Ammermuller, 1999; Witkovsky, 2000; Witkovsky & Stone, 1983). It is crucial, therefore, to conduct a systematic study on synaptic connections of all types of photoreceptors in order to determine how visual signals are conveyed to various types of inner retinal neurons.
Glutamate is a major excitatory neurotransmitter in the retina. There are two types of glutamate receptors: G-protein linked metabotropic and ligand-gated ionotropic. Ligand-gated ionotropic glutamate receptors are further divided into AMPA/kainate and NMDA receptors. AMPA receptors consist of four subunits: GluR1, GluR2, GluR3, and GluR4 (Grünert, Haverkamp, Fletcher, & Wassle, 2002; review see Brandstatter, Koulen, & Wassle, 1998). Earlier physiological studies showed that in the lower vertebrate retina postsynaptic responses of OFF-bipolar cells and horizontal cells are mediated by AMPA-preferring receptors (Yang, Maple, Gao, Maguire, & Wu, 1998; Cadetti, Tranchina, & Thoreson, 2005). However, it is not clear which subunits of AMPA receptors mediate synaptic transmission between photoreceptors and second order neurons. There is a study showing that glutamate receptors differ in rod- and cone-dominant OFF-bipolar cells (Maple, Gao, & Wu, 1999), indicating that different subunits of AMPA receptors may function at rod or cone synapses. Immuocytochemical studies in the goldfish retina demonstrate that GluR2 and GluR4 are expressed postsynaptically at photoreceptor synapses and are differentially located at bipolar and horizontal dendrites (Klooster, Studholme, & Yazulla, 2001; Yazulla & Studholme, 1999). Therefore, morphological study on the AMPA receptors in the outer plexiform layer (OPL) of the salamander retina is warranted.
In the present study, we used the immunocytochemical technique in conjunction with confocal microscopy to examine the morphology of salamander rod and cone photoreceptors and to examine the differential connection between rods/cones and bipolar/horizontal cells in the outer retina. In addition, we elucidated the wide distribution of AMPA receptor subunits GluR2/3 and GluR4 at the first visual synapses. Our results allow a comprehensive incorporation of the anatomic features with the physiological properties of photoreceptor synapses in the salamander retina, and possible neurochemical circuitry of photoreceptor-bipolar/horizontal cell synapses is discussed.
2. Methods
2.1. Preparation
Larval tiger salamanders, Ambystoma tigrinum, (Charles D. Sullivan Co., Nashville, TN) were maintained on a daily 12-h light/dark cycle. The University Committee on Animal Use at Baylor College of Medicine approved the use of animals and all animals were treated in accordance with NIH guidelines. The animals were decapitated and the eyes were enucleated and hemisected. The cornea, lens and vitreous were removed. The retinas were removed from the posterior eyecups and flattened onto a cover glass. For the wholemount preparations, the retinas were fixed in fresh 4% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4) for 30–60 minutes at room temperature. Following fixation, the retinas were extensively rinsed with PBS for further immuno-processing.
2.2. Immunocytochemistry
For double-labeling experiments, wholemount retinal tissues or free floating sections were blocked with 3% donkey serum in PBS with 0.5% Triton X-100/0.1% sodium azide for 2 h to overnight to reduce nonspecific labeling. The tissues were incubated in primary antibodies in the presence of 1% donkey serum/PBS with 0.5%Triton X-100/0.1% sodium azide for 5–10 days at 4 °C. Controls lacking primary antibodies were also processed. Following extensive washing with PBS containing 0.5% Triton X-100/0.1% sodium azide, the tissues were incubated overnight with immunofluorescent secondary antibodies. After extensive rinsing, the tissues were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and immunofluorescence was visualized by confocal laser-scanning microscopy (Zeiss LSM 510, Zeiss, NY).
2.3. Antibody
Primary antibody in a 1:1000 dilution of polyclonal rabbit anti-sera against glutamate (antibody# AB133; Chemicon International, Temecula, CA) was used in this study. The specificity of antibodies against glutamate has been documented in the manufacturer's Data Sheet. A rabbit antiserum against the calcium-binding protein recoverin isolated from bovine retinal photoreceptors in a dilution of 1:1000 was kindly provided by Dr. A.M. Dizhoor (Pennsylvania College of Optometry, Elkins Park, PA; Dizhoor et al., 1991) and used to label photoreceptors of human, macaque monkey, rat, ground squirrel, and salamander retinas (Dizhoor et al., 1991; Von Schant, Szel, vanVee, & Farber, 1994; Zhang & Wu, 2003). The mouse monoclonal antiserum against calcium-binding protein calbindin D28K purchased from SWant (Code#300) was used in a dilution of 1:1000. Goat polyclonal antibody against calcium-binding protein calretinin (Code# CG1; SWant, Bellinzona, Switzerland) was used in a dilution of 1:1000. Antibodies against the calcium-binding protein calretinin and calbindin D28K have been shown in the studies of the amphibian retina to label photoreceptors and horizontal cells (Deng et al., 2001; Hamano et al., 1990; Pasteels, Rogers, Blachier, & Pochet, 1990). Antibodies against S-cone opsin were used at a dilution of 1:200 (kindly provided by Dr. J. Ma, University of Okalahoma) and used to label salamander small rods and blue sensitive cones (Ma et al., 2001). Antibodies against synaptic vesicle proteins (SV2, Developmental Studies Hybridoma Bank at the University of Iowa) and ionotropic glutamate AMPA receptors (GluR2/3, antibodies # AB1506, and GluR4, antibodies # AB1508, Chemicon) were used at dilution of 1:5000, 1:500 and 1:100, respectively. The specificity of antibodies against SV2, GluR2/3 and GluR4 has been documented in the manufacturer's Data Sheet. The same antibodies have been used to label the synaptic vesicle proteins and glutamate AMPA receptors in photoreceptors and bipolar/horizontal cells of the salamander and cat retinas (Morigiwa & Vardi, 1999; Vardi, Morigiwa, Wang, Shi, & Sterling, 1998; Zhang & Wu, 2003; Zhang, Yang, & Wu, 2004). Appropriate donkey conjugated CY3 and Alexa 488 secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA) and Molecular Probes and were used in a dilution of 1:100.
2.4. Data analysis
The fluorescent specimens were visualized with a Zeiss LSM 510 confocal laser-scanning microscope (Zeiss, NY). Images were acquired using 40× and 63× oil-immersion objective lenses and were scanned with 1024 × 1024 pixels. The brightness and contrast of the images were adjusted using Adobe Photoshop 5.0. For each set of wholemount experiments, 2–4 whole retinas triple-labeled with different antibodies were imaged and the data were stored and used for off-line analysis. All cells were counted manually in this study. The cell density and distribution pattern were analyzed using Microsoft Excel 98 and Origin 7.0 and presented as means ± SEM.
3. Results
3.1. Photoreceptor subtypes in the tiger salamander retina
In the vertical sections as shown in Fig. 1A, antibodies against glutamate strongly labeled somas of cones (C) which lay in the inner tier of the outer nuclear layer (ONL), and those of rods (R) which lay in the outer tier of the ONL. Six morphologically distinct types of rods/cones were readily distinguishable. Large rods (LR, Fig. 1B1), with large inner/outer segments in the outer segment layer (OSL), had narrowed axons connecting the synaptic ending (rod pedicles) (arrowheads) in the OPL. Small rods (SR) with longer and narrower inner segments had their somas extending from the rod layer nearly to the OPL, without a significant intervening axon segment separating the synaptic ending (arrowhead, Fig. 1B2). Two types of single cones were identified: the large single cones with round to egg-shaped somas (LSC, Fig. 1C1), and small single cones (SSC) that had more elongated oval-shaped somas and synaptic pedicles (arrows) adjacent to the somas (Fig. 1C2). Moreover, tightly closed double cones (DC) including the principal member (pc, arrow) and the accessory member (ac, dual arrows) were identified based on the shapes of their outer segments and pedicles (Fig. 1C3).
Fig. 1.
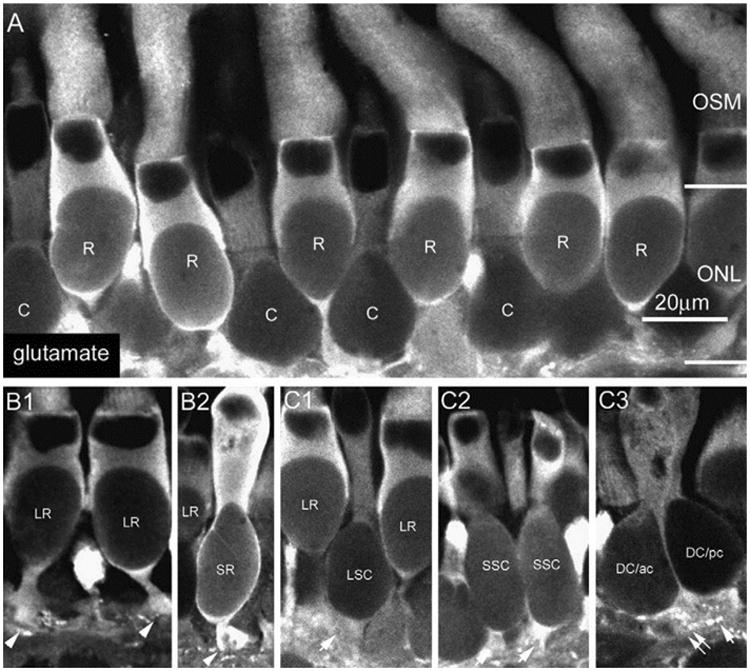
In the vertical sections, glutamate strongly labeled somas of cones (C) and rods (R) which lay in the ONL (A). Six morphologically distinct types of rods/cones were observed: large (red) rods (LR) (B1), small (green) rods (SR) (B2), large single cone (LSC) (C1), small single cones (C2), and tightly closed double cones (DC) including the principal member (pc, arrow) and the accessory member (dual arrows) (C3).
Our previous study showed that recoverin antibodies weakly label rods and strongly label cones in the salamander retina (Zhang & Wu, 2003). In this study, as shown in Fig. 2E, we found that the majority of cones were immunoreactive not only to recoverin antibodies (shown in green) but also to calbindin antibodies (shown in red) (Fig. 2E). Particularly, when the images were taken at the focal plane of the OSL or the ONL (Fig. 2A and B) of the wholemount retina, calbindin was found to profoundly stain the accessory members of double cones (ac) and weakly stain single cones and the principal members of double cones (pc). In the vertical section, calbindin immunoreactive accessory cones had uniquely small pedicles (dual arrows, Fig. 2C) and outer segments, which have the morphology that corresponds to Mariani's accessory cones (1986). In the double-labeled wholemount salamander retina as shown in Fig. 2D (at the level of the OSL) & 2E (at the level of the ONL), it was evident that calbindin (red) positive accessory members (ac) of double cones were also immunoreactive to recoverin antibodies (green). We examined a total of 4 wholemount retinas and found that the average density of accessory double cones was 406 ± 25 cells/mm2. Since the average density of salamander cones is 3526 ± 908 cells/mm2 and the number of principal members of double cones was the same as the accessory double cones, the total number of principal and accessory members of double cones accounted for about 23% of total cones in the salamander retina.
Fig. 2.
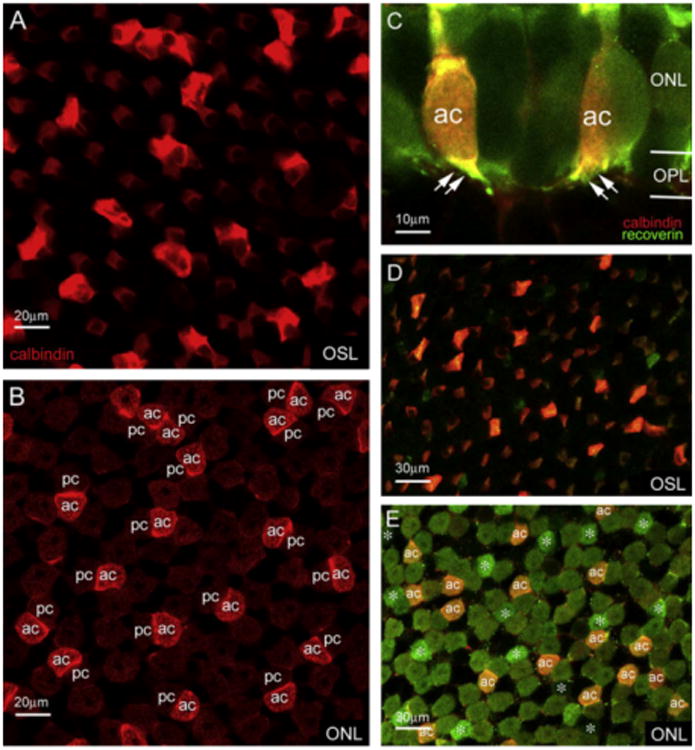
The accessory members of double cones were strongly immunoreactive to calbindin antibodies as shown in the outer segment layer (OSM) (A) or in the ONL (B). Accessory cones positively labeled with calbindin had unique pedicles (dual arrows) and was confirmed in the double-labeled retinal section and wholemount with calbindin (red) and recoverin (green) antibodies (C-E). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this paper.)
3.2. S-Cone opsin immunoreactivity in the salamander retina
A small population of photoreceptors was immunoreactive to S-cone opsin antibody. In the single vertical sections as illustrated in Fig. 3A1 and A2, three types of photoreceptors were found to be S-cone opsin positive: small rods (arrowhead), small single cones (arrow), and cone outer segments (dual arrows). The stacked con-focal images of small rods and small single cones are shown in Fig. 3B and C, respectively. The morphological properties and spatial distribution of S-cone opsin positive rods/cones is shown in the wholemount retina, where the images were taken at the levels of the OSL (Fig. 3D1), the ONL (Fig. 3D2), and the OPL (Fig. 3D3). In addition, all S-cone opsin positive small rods and small single cones exhibited radially oriented teleodendrites that originated from their synaptic endings (Fig. 4). However, we noticed that Scone opsin positive teleodendrites did not cover the entire OPL. While the tip-to-tip teleodendritic contacts between S-cone opsin positive small rods (arrowheads) and between S-cone opsin positive small single cones (arrows) were observed (Fig. 4A), sometimes there was only a small single cone (arrow) (or small single rods) in the area (Fig. 4B). Tip-to-tip teleodendritic contacts between S-cone opsin positive small rods (arrowheads) and S-cone opsin positive small single cones (arrows) were also found as shown in Fig. 4C and D.
Fig. 3.
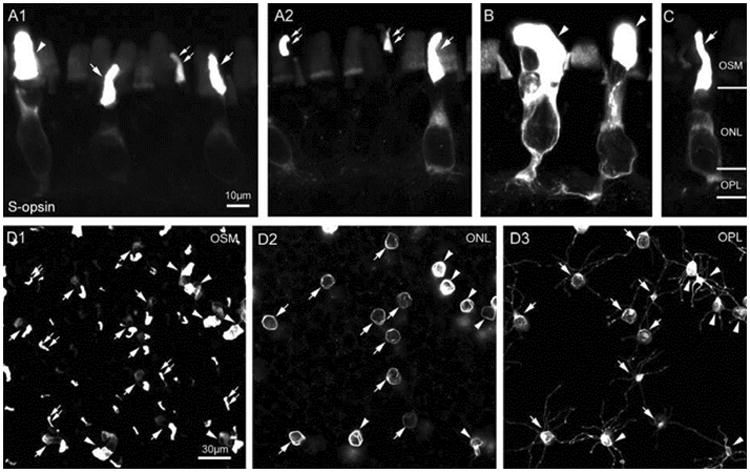
A small population of cones was immunoreactive to S-opsin antibody. In the single vertical sections (A1 and A2), three types of photoreceptors were identified: small rods (SRs) (arrowhead), small single cones (SSCs) (arrow), and single cone outer segments (dual arrows). The stacked images of the morphologies of SR and SSC are shown in B and C. In the wholemount retina, all SRs and SSCs with focal planes at the level of the OSM (D1), the ONL (D2), and the OPL (D3) exhibited radially oriented teleodendrites that originated from their synaptic endings (D3).
Fig. 4.
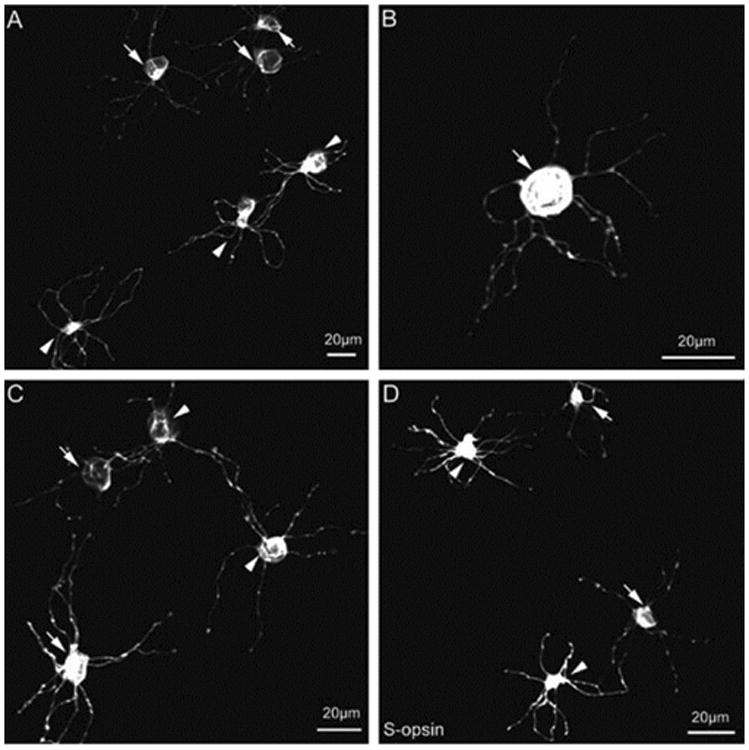
S-opsin positive teleodendrites did not cover the entire OPL: the tip-to-tip teleodendritic contacts between SRs (arrowheads) and between SSCs (arrows) (A), only SSC (arrow) (or SR) in the area (B), and tip-to-tip teleodendritic contacts between SRs (arrowheads) and SSCs (arrows) were found (C and D).
It was evident in the vertical sections that a subgroup of S-cone opsin positive outer segments (blue, arrows) belonged to calbindin positive accessory cones (red, dual arrows) (Fig. 5A). In the whole-mount retina, outer segments of accessory cones were found to be S-cone opsin positive (arrows) (Fig. 5B). We examined 4 wholemount retinas and counted about 50,000 calbindin positive accessory double cones. We found that all outer segments of accessory cones were S-cone opsin positive. Therefore, one subpopulation of S-cone opsin positive outer segments was actually accessory double cones. In contrast, the outer segments of principal double cones were S-cone opsin negative. Yet the identity of the remainder of S-cone opsin positive outer segments was still uncertain. It should be noticed that in the same field, the rod-shaped outer segment (blue, arrowhead) was from a small rod. Moreover, as shown in the wholemount retina at the levels of the ONL (Fig. 5C) and the OPL (Fig. 5D), it was obvious that S-cone opsin positive small rods (R) and small single cones (C) were not co-localized with calbindin positive accessory cones.
Fig. 5.
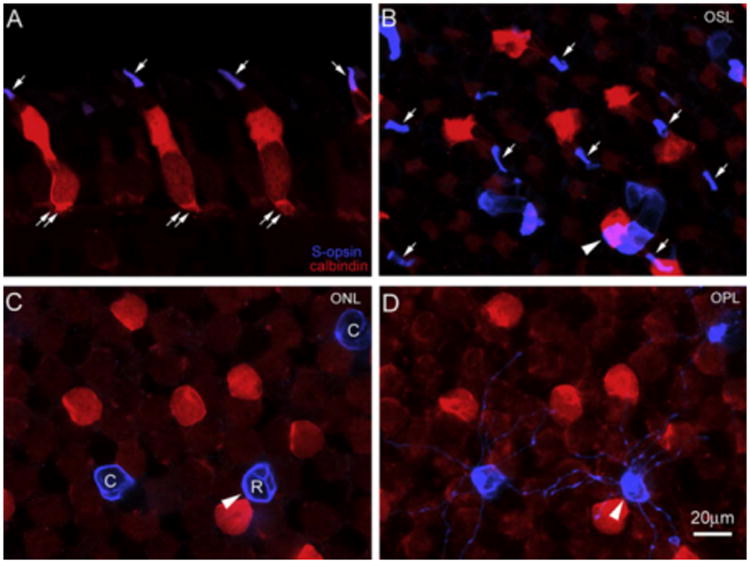
A subgroup of S-opsin positive outer segments (arrows, blue) belonged to calbindin positive accessory cones (dual arrows, red) (A). In the wholemount retina, all outer segments of accessory cones were found to be S-opsin positive (arrows) (B). In the same field, S-opsin positive SRs and SSCs were not co-localized with calbindin positive accessory cones in C (ONL) and D (OPL). The rod-shaped outer segment (blue, arrowhead) was from a SR (C). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this paper.)
3.3. Spatial distribution of S-cone opsin positive photoreceptors
We next examined the spatial distribution of S-cone opsin positive photoreceptors and their densities in five wholemount retinas. We found that while the density of accessory members of double cones was reasonably consistent across the retina, the densities of S-cone opsin positive small rods and small single cones gradually varied from the temporal to the nasal area. Fig. 6A shows the spatial distributions of the three types of photoreceptor from a typical salamander retina. We observed a density gradient along the temporal-nasal meridian (Fig. 6B): small rods had a higher density in the temporal than in the nasal area, whereas small single cones had a lower density in the temporal than in the nasal area. On average, counting from the total of 5 retinas, the average density of total S-cone opsin positive outer segments was 821 ± 25 cells/mm2, of which the average densities of small rods and small single cones, S-cone opsin positive unidentified single cones, and S-cone opsin positive accessory double cones were 61 ± 10, 157 ± 17, 197 ± 49, and 406 ± 25 cells/mm2, respectively. In the salamander retina, the density of rods was 4400 ± 1134 cells/mm2 and for cones was 3526 ± 908 cells/mm2 (Zhang & Wu, 2003). Therefore, S-cone opsin positive small rods accounted for about 1.4% of total rods, whereas S-cone opsin positive small single cones, unidentified single cones and accessory double cones accounted for 4.5%, 5.6%, and 11.5% of total cones in the salamander retina (Fig. 6C).
Fig. 6.
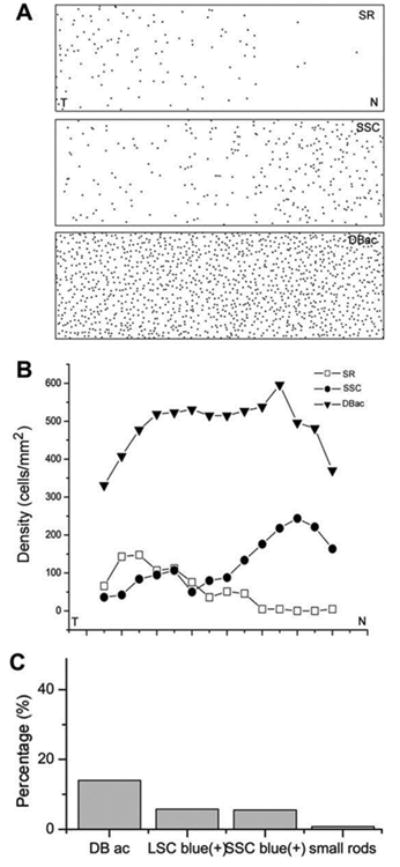
(A) The map of small rods (SR), small single cones (SSC), and accessory members of double cones (DBac) across the retina from the nasal to temporal area. (B) The spatial distribution of cell densities of SRs, SSCs and DBac across the retina from the nasal to temporal area. (C) The percentage of different types of S-opsin positive cells in the total number of cones in the salamander retina.
3.4. Photoreceptor synaptic contacts with bipolar and horizontal cells in the outer plexiform layer
We previously showed that rod and cone terminals in the tiger salamander retina contained synaptic vesicle proteins (SV2) (Zhang & Wu, 2003), providing evidence of chemical synapses involved in the first visual synapse. In this study, we also showed that the characteristic punctal staining of glutamate AMPA receptors GluR2/3 and GluR4 (green) was present in this first visual synapse. They were located just underneath SV2 positive rod/cone synapses (red) as illustrated in the vertical sections (Fig. 7A and B). In the wholemount retina, GluR2/3 appeared as discrete puncta deposited at the pocket-like rod/cone synapses (Fig. 7C), but GluR4 formed clustered puncta mostly centered in the middle of cone pedicles (Fig. 7D). This observation was made from all the retinas we examined. These data suggest that AMPA receptors play an important role in glutamatergic neurotransmission at the first visual synapses in the salamander retina.
Fig. 7.
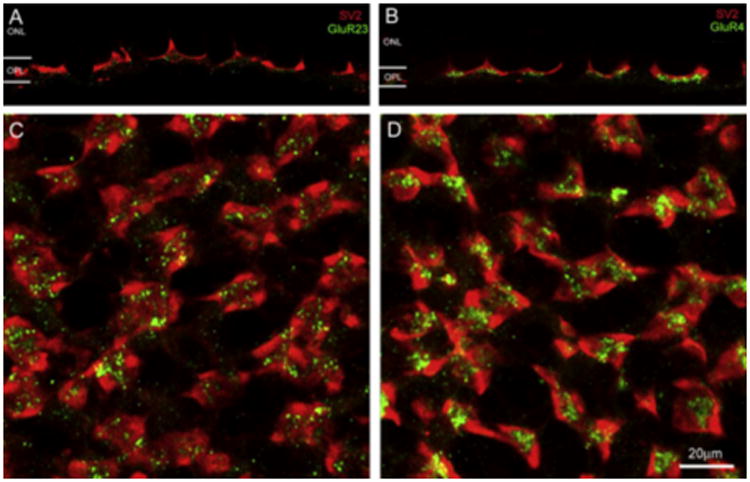
In the vertical section, glutamate AMPA receptors GluR2/3 and GluR4 (green) were located underneath the rod/cone synapses labeled by SV2 antibodies (red) (A and B). In the wholemount retina, GluR2/3 appeared as discrete puncta associated with rod/cone synapses (C), but GluR4 formed clustered puncta mostly centered in the middle of cone pedicles (D). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this paper.)
We also conducted double-labeling experiments on vertically sectioned retinas and wholemount retinas to reveal the detailed morphology of S-cone opsin positive small rods and small single cones' synaptic endings in the OPL. In the wholemount retina double-labeled with S-cone opsin and SV2 antibodies, the synaptic terminals of both small rods and small single cones were revealed by S-cone opsin (blue, Fig. 8A (top panel)), whereas all rod/cone pedicles were labeled by SV2 (red, Fig. 8A (middle panel)). When two images were superimposed (Fig. 8A (bottom panel)), the terminals of S-cone opsin positive small rods and small single cones were found registered immediately to the SV2 labeled synapses (arrowheads), suggesting that both S-cone opsin positive small rods and small single cones use the chemical synapses for glutamate neurotransmission. This observation was also consistent with the results obtained from the retinal sections double-labeled with the two antibodies (Fig. 8B), where the co-localization of SV2 with S-cone opsin positive small rods (top & middle panels) and small single cones' synaptic endings (bottom panels) was evident.
Fig. 8.
The detailed morphology of SRs and SSCs' synaptic endings in the OPL were revealed. In the wholemount retina double-labeled with S-opsin and SV2, both SRs and SSCs's terminals (blue, top, A) contained SV2 (red, middle, A) at their synaptic endings (bottom, A). In the retinal sections double-labeled with the two antibodies, the co-localization of SV2 with SRs (top and middle, B) and SSCs' (bottom, B) synaptic endings was also evident. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this paper.)
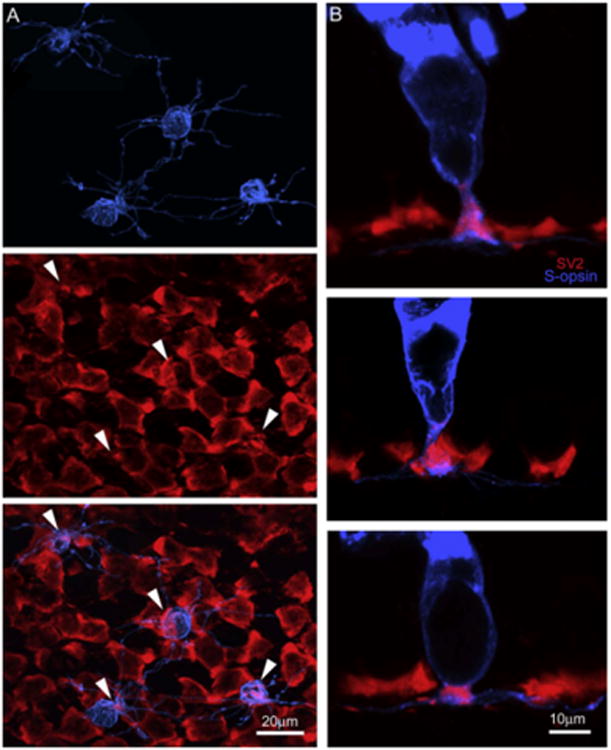
In the salamander retina, there are at least 12 types of bipolar cells (Wu et al., 2000). Among them, subpopulations of ON cone bipolar cells (BCs) have been previously characterized by using G0αantibodies (Zhang & Wu, 2003). In the present study, we used this antibody to label a subpopulation of ON cone bipolar cells (BCs) showing dendrites that formed clusters in the wholemount retina (Fig. 9A, left panel). By carefully examining the double label stacked confocal images, we found that S-cone opsin positive small single cones (arrow) and small rods (arrowhead) (blue) made many contacts with G0α positive ON BC processes (red). In the vertical sections, the examples of S-cone opsin positive small single cones contacting G0α positive ON BC processes are shown in the middle two panels and S-cone opsin positive small rods contacting those are shown in the right two panels in Fig. 9A. A contact here is where processes from two neurons closely appose each other at specialized areas known from electron microscopy to typically form synaptic connections. For example, when the thick part of a BC dendrite travels next to a photoreceptor, a synapse is unlikely, but when a BC dendritic cluster is nestled up against the underside of a photoreceptor terminal, synaptic contact is expected based on EM anatomical correlation. These results suggest that subtypes of G0α positive ON BCs might receive inputs from S-cone opsin positive small rods and small single cones.
Fig. 9.
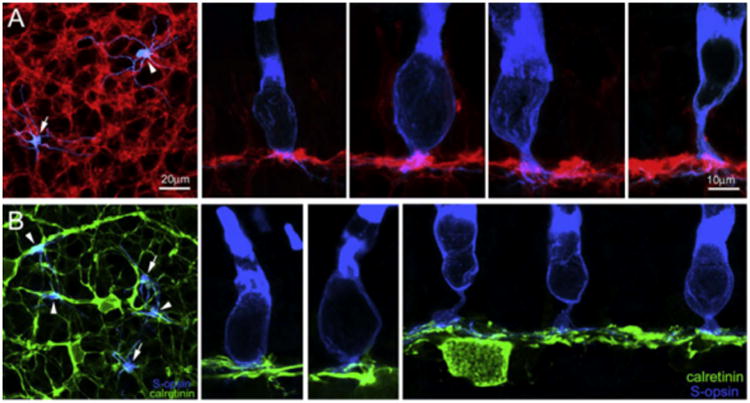
In the retinal wholemounts and sections double-labeled with S-opsin (blue) and Goa (red) (A1–A5), Goa-positive processes were found contacting SSCs (A1, A2, A3) and SRs (A1, A4, A5). In the retinal wholemounts and sections double-labeled with S-opsin (blue) and calretinin (green) (B1–B4), calretinin-positive processes were found contacting SSCs (B1, B2, B3) but not SRs (B1, B4). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this paper.)
A- and B-types of horizontal cells (HCs) in the tiger salamander retina can be identified by calretinin and GABA antibodies, respectively (Zhang, Zhang, & Wu, 2006a; Zhang, Zhang, & Wu, 2006b). In particular, along its whole length of 100–200 μm, the dendrites of calretinin-positive A-type HCs are decorated by irregularly spaced small bushy-like aggregates or clusters that were unique to the tiger salamander, and are where synaptic interaction is likely to occur. In this study, we also carried out double-labeling experiments using calretinin (green) and S-cone opsin (blue) antibodies in the wholemount retina (Fig. 9B, left panel). S-cone opsin positive small rods were found not to contact these calretinin-positive dendritic clusters (arrowheads), only teminating near non-specialized areas of the HC processes. But, S-cone opsin positive small single cones (arrows) were found to contact calretinin-positive dendritic clusters. Vertical sections were also used to reveal the relationship between S-cone opsin positive small single cones (Fig. 9B, the middle two panels)/small rods (Fig. 9B, right panel) and calretinin-positive dendritic processes. These data suggest A-type HCs receive some inputs from S-cone opsin positive small single cones and do not receive inputs from S-cone opsin positive small rods in the tiger salamander retina.
4. Discussion
4.1. Photoreceptors and their subpopulations
Our immunocytochemical data on glutamate and recoverin labeling demonstrate the six morphologically distinct types of photoreceptors in the salamander retina, which includes large and small rods, large and small single cones, and double cones composed of principal and accessory members. Among them, the vast majority of rods and cones are immunoreactive to antibodies against calbindin, whereas a small amount of them are immunoreactive to antibodies against S-cone opsin. S-cone opsin positive photoreceptors include small rods, a subpopulation of small single cones, the outer segments of accessory double cones and a subtype of unidentified single cones. On average, large rods and small Scone opsin positive rods accounted for 98.6% and 1.4% of all rods, respectively. Large and small cones, principle and accessory double cones, S-cone opsin positive small single cones, and S-cone opsin positive unidentified single cones accounted for about 66.9%, 23%, 4.5%, and 5.6% of the total cones, respectively. The percentage value of various antibodies-positive rods and cones is summarized in Table 1.
Table 1.
Summary of percentage value of rods and cones labeled by various antibodies.
| Antibodies | Large rods | Small rods | Large single cones | Small single cones | Principle double cones | Accessory double cones | S-cone opsin positive cones | % of total rods | % of total cones |
|---|---|---|---|---|---|---|---|---|---|
| Glutamate | +++ | +++ | +++ | +++ | +++ | +++ | 100.00 | 100.00 | |
| Recoverin | ++ | ++ | +++ | +++ | +++ | +++ | 100.00 | 100.00 | |
| Calbindin | +++ | ++ | +++ | ++++ | 0.00 | 90.00 | |||
| S-cone opsin | ++++ | ++++ | +++ | 10.10 | |||||
| % of total rods | 98.60 | 1.40 | |||||||
| % of total cones | 66.90 | 11.50 | 11.50 | 10.10 |
These results are consistent with physiological evidence demonstrating that the light responses of the majority of cones are sensitive to red light, whereas some cones are sensitive to blue light (Makino & Dodd, 1996; Perry & McNaughton, 1991; Stella & Thoreson, 2000). It is also consistent with the morphological studies showing that a subpopulation of salamander rods and cones contained S-cone opsin pigments (Ma et al., 2001; Sherry, Bui, & Degrip, 1998; Xu, Hazard, Lockman, Crouch, & Ma, 1998). Although we do not know the mechanisms of why S-cone opsin is expressed in small rods, photocurrent measurements illustrate that S-cone opsin positive small rods are sensitive to blue flashing-light (Ma et al., 2001), suggesting that this subtype of rods may behave like blue sensitive cones.
By comparing the double-labeling results of S-cone opsin and calbindin immunostaining, we found that accessory members of double cones are S-cone opsin positive. This subpopulatdion of cones accounts for about 11.5% of total cones in the salamander retina. This result is somewhat surprising because early physiological studies suggest that the two halves of double cones are not electrically coupled and the spectral sensitivity of both halves is maximum around 620 nm wavelength (Attwell et al., 1984). There are several possible explanations for our observation. Firstly, it may be attributed to the fact that polyclonal S-cone opsin antibody was used in this study, which might cause nonspecific labeling in our experiments. However, the specificity of this antibody was tested in the earlier study done by Ma and his colleagues (Ma et al., 2001). Thus, it likely rules out the possibility of nonspecific labeling of S-cone opsin in the accessory members of double cones. Or accessory double cones may carry more than one opsin. Both red and S-cone opsin may be present in the double cones and function differently. In an early physiological study, a case of a cone carrying two visual pigments in the salamander retina was reported (Makino & Dodd, 1996). In order to better understand why accessory double cones possess S-cone opsin and whether the accessory members of double cones are sensitive to both red and blue lights needs to be further examined.
4.1.1. Expression of glutamate AMPA receptors in the OPL
By using single- and double-immunostaining techniques, we have shown that both AMPA receptor subunits GluR2/3 and GluR4 were abundantly expressed in the OPL. In addition, the spatial distribution of AMPA receptor subunits GluR2/3 and GluR4 was rather different from each other: GluR4 subunits tend to cluster tightly underneath the cone pedicles, whereas GluR2/3 tend to sparsely associate with the rod and cone pedicles. The enhanced staining of GluR4 in the cone pedicles indicates that GluR4 is possibly involved more in cone vision than in rod vision, whereas GluR2/3 may have no preference for either rod or cone vision. Although we could not visualize synaptic vesicles and other ultrastructural synaptic features, electron microscopic studies have shown that most rod and cone-horizontal cell synapses are made in the finger-shaped dendritic clusters or invaginations in the rod and cone pedicles (Lasansky, 1973, 1978; Mariani, 1986). This feature was also observed in our recent immunohistochemistry study of horizontal cells in the same species (Zhang et al., 2006a; Zhang et al., 2006b). Therefore, our data imply that GluR4 is possibly expressed in the dendritic processes of horizontal cells. Electron microscopic studies have shown that bipolar cells, however, often make the basal junctional types of connections with rod and cone pedicles. It seems reasonable to assume that GluR2/3 in the salamander retina is likely to be expressed in OFF cone bipolar cell dendrites. Yet, the possibility of co-expression of GluR2/3 and GluR4 in the same cell type still exists. Moreover, the staining pattern of GluR2/3 and GluR4 was quite uniform across the wholemount retina and no missing elements at particular rod and cone pedicles were found in our experiment. This observation indicates that there may be no difference between S-cone opsin positive small rods/small single cones and other majorities of rods and cones in the expression of AMPA receptors. In summary, our data suggest that AMPA receptors GluR2/3 and GluR4 play an important role in the first visual synapses. Both OFF-bipolar and horizontal cells may utilize AMPA receptors for glutamatergic synaptic transmission. This anatomical feature is consistent with the physiological finding described in earlier studies (Cadetti et al., 2005; Yang et al., 1998).
4.2. Selective interconnections between second order neurons and photoreceptors
In the tiger salamander retina, there are at least 12 subtypes of bipolar cells, which can be generally classified as rod-dominant ON- and OFF-bipolar cells, or cone-dominant ON- and OFF-bipolar cells (Wu et al., 2000). There are also two morphologically distinct horizontal cells present: cone-dominant A-type horizontal cells and rod-cone mixed B-type horizontal cells (Zhang et al., 2006a; Zhang et al., 2006b). Our data suggest that the synaptic connections between subtypes of bipolar/horizontal cells and subtypes of rods and/or cones are more complicated than previously recognized. Here, we qualitatively summarize our results in Table 2, which combines data from this study with results from previous reports.
Table 2.
Qualitative summary of contacts between rods/cones and bipolar/horizontal cells.
| Cell types | Large rods | Small rods | Large single cones | Small single cones | Principle double cones | Accessory double cones | S-cone opsin positive small single cones | S-cone opsin positive unidentified single cones |
|---|---|---|---|---|---|---|---|---|
| HCA | + | + | ++ +++ | + | ||||
| HCB | ++++ | ++ | ++++ + | ++ | +++++ | + | ||
| DBCR | +++++ | +++ | + | + | + | + | + | + |
| DBCC | + | + | ++++ + | +++ | +++ ++ | ++ | ++ | ++ |
| HBCR | +++++ | +++ | + | + | + | + | + | + |
| HBCC | + | + | ++++ + | +++ | +++ ++ | ++ | ++ | ++ |
A-type and B-type of horizontal cells have their own unique contacts with rods and/or cones. Our recent study shows the GABA-positive B-type horizontal cells postsynaptic to rods, large single cones (LSC), small single cones (SSC) and principal double cones (PDC) (also seen in Fig. 10) (Zhang et al., 2006a; Zhang et al., 2006b). However, this wiring pattern is not applied to A-type horizontal cells. A good example from this study is that the calretinin-positive A-type horizontal cells were selectively postsynaptic contacting S-cone opsin/calbindin positive accessory members of double cones (ADC). Furthermore, A-type horizontal cells do not make synaptic contacts with S-cone opsin positive small rods (SR) (Fig. 9). On average, we showed that 100% of accessory double cones and only 6% of the principal double and single cones contact A-type horizontal cell dendrites (Zhang et al., 2006a; Zhang et al., 2006b). Therefore, it seems that the majority of inputs to A-type horizontal cells are from accessory double cones but not from Scone opsin positive small rods or small single cones. At present, although we are uncertain whether accessory double cones carry both red and blue visual pigments, the contacts between accessory members of double cones and A-type horizontal cells is fairly unique to the salamander retina.
Fig. 10.
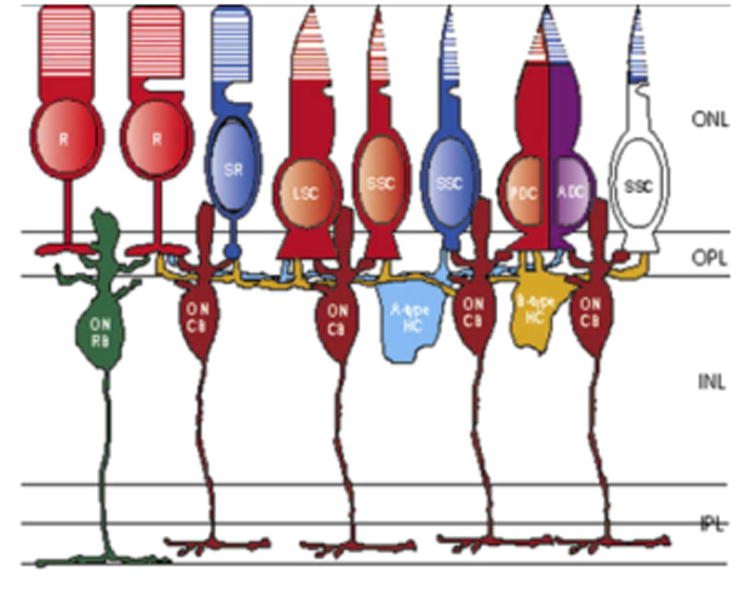
A schematic diagram of the first visual synapses in the salamander retina. Subtypes of bipolar and horizontal cells may target different types of rods and cones. R: rods; SR: small rods; LSC: large single cones; SSC: small single cones; PDC: principal members of double cones; ADC: accessory members of double cones; ON RB: rod-dominant ON bipolar cells; ON CB: cone-dominant ON bipolar cells; A-type HC: A-type horizontal cells; B-type HC: B-type horizontal cells. ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer. Red: red pigment; blue: blue pigment; purple: red/blue pigment; white: unidentified blue sensitive cones. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this paper.)
Early electrophysiological studies have shown that, in the salamander retina, rod-dominant bipolar cells (RB, Fig. 10) primarily receive inputs from rods, whereas cone-dominant bipolar cells mainly receive inputs from cones and the remaining bipolar cells receive inputs from both rods and cones (Fig. 10, also see Wu et al., 2000; Yang & Wu, 1997). Here, we further showed that GOα positive ON bipolar cells (ON CB) were postsynaptic to S-cone opsin positive small rods (SR) and small single cones (SSC). Because of the small population of S-cone opsin positive small rods and small single cones, we anticipate that some, but not all, subtypes of ON bipolar cells may make synaptic contacts with S-cone opsin positive small rods and/or S-cone opsin positive small single cones. Since the specific antibodies against blue cone bipolar cells are not currently available for the salamander retina, it is uncertain whether blue cone bipolar cells exist as they do in the primate retinas (Kouyama & Marshak, 1992). In summary, our results show that the different photoreceptors selectively contact bipolar and horizontal cells, which supports the idea that visual signals may be conveyed to the inner retina by different functional pathways in the outer retina.
Acknowledgments
We thank Dr. A.M. Dizhoor for providing recoverin antibodies, and Dr. Roy Jacoby for critically reading the manuscript.
Grant Sponsor: National Institute of Health; Grant number: EY 04446, EY 02520 (core grant); the Retina Research Foundation (Houston), and the Research to Prevent Blindness, Inc.
References
- Attwell D, Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. Journal of Physiology. 1980;309:287–315. doi: 10.1113/jphysiol.1980.sp013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Wilson M, Wu SM. A quantitative analysis of interactions between photoreceptors in the salamander (Ambystoma) retina. Journal of Physiology. 1984;352:703–737. doi: 10.1113/jphysiol.1984.sp015318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstatter JH, Koulen P, Wassle H. Diversity of glutamate receptors in the mammalian retina. Vision Research. 1998;38:1385–1397. doi: 10.1016/s0042-6989(97)00176-4. [DOI] [PubMed] [Google Scholar]
- Cadetti L, Tranchina D, Thoreson WB. A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina. Journal of Physiology. 2005;569(3):773–788. doi: 10.1113/jphysiol.2005.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Cuenca N, Doerr T, Pow DV, Miller R, Kolb H. Localization of neurotransmitters and calcium binding proteins to neurons of salamander and mudpuppy retinas. Vision Research. 2001;41:1771–1783. doi: 10.1016/s0042-6989(01)00060-8. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, et al. Recoverin: A calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The retina, an approachable part of the brain. Harvard University Press; 1987. [Google Scholar]
- Grünert U, Haverkamp S, Fletcher EL, Wassle H. Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. Journal of Comparative Neurology. 2002;447:138–151. doi: 10.1002/cne.10220. [DOI] [PubMed] [Google Scholar]
- Hamano K, Kiyama H, Emson PC, Manabe R, Nakauchi M, Tohyama M. Localization of two calcium binding proteins, calbindin (28 kDa) and parvalbumin (12 kDa), in the vertebrate retina. Journal of Comparative Neurology. 1990;302:417–424. doi: 10.1002/cne.903020217. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Mockel W, Ammermuller J. Different types of synapses with different spectral types of cones underlie color opponency in a bipolar cell of the turtle retina. Visual Neuroscience. 1999;16:801–809. doi: 10.1017/s0952523899164186. [DOI] [PubMed] [Google Scholar]
- Klooster J, Studholme KM, Yazulla S. Localization of the AMPA subunit GluR2 in the outer plexiform layer of goldfish retina. Journal of Comparative Neurology. 2001;441:155–167. doi: 10.1002/cne.1404. [DOI] [PubMed] [Google Scholar]
- Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. Journal of Neuroscience. 1992;12:1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1973;265:471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Lansansky A. Contacts between receptors and electrophysiologically identified neurons in the retina of the larval tiger salamander. J Physiol. 1978;285:531–542. doi: 10.1113/jphysiol.1978.sp012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Znoiko S, Othersen KL, Ryan JC, Das J, Isayama T, et al. A visual pigment expressed in both rod and cone photoreceptors. Neuron. 2001;32:451–461. doi: 10.1016/s0896-6273(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Makino CL, Dodd RL. Multiple visual pigments in a photoreceptor of the salamander retina. The Journal of General Physiology. 1996;108:27–34. doi: 10.1085/jgp.108.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple BR, Gao F, Wu SM. Glutamate receptors differ in rod- and cone-dominated off-center bipolar cells. Neuroreport. 1999;10:3605–3610. doi: 10.1097/00001756-199911260-00026. [DOI] [PubMed] [Google Scholar]
- Mariani AP. Photoreceptors of the larval tiger salamander retina. Proceedings of Royal Society of London B: Biological Science. 1986;227:483–492. doi: 10.1098/rspb.1986.0035. [DOI] [PubMed] [Google Scholar]
- Morigiwa K, Vardi N. Differential expression of ionotropic glutamate receptor subunits in the outer retina. Journal of Comparative Neurology. 1999;405:173–184. doi: 10.1002/(sici)1096-9861(19990308)405:2<173::aid-cne3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Pasteels B, Rogers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Visual Neuroscience. 1990;5:1–16. doi: 10.1017/s0952523800000031. [DOI] [PubMed] [Google Scholar]
- Perry RJ, McNaughton PA. Response properties of cones from the retina of the tiger salamander. Journal of Physiology. 1991;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Bui DD, Degrip WJ. Identification and distribution of photoreceptor subtypes in the neotenic tiger salamander retina. Visual Neuroscience. 1998;15:1175–1187. doi: 10.1017/s0952523898156201. [DOI] [PubMed] [Google Scholar]
- Stella SL, Jr, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. European Journal of Neuroscience. 2000;12:3348–3537. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- Vardi N, Morigiwa K, Wang TL, Shi YJ, Sterling P. Neurochemistry of the mammalian cone ‘synaptic complex’. Vision Research. 1998;38:1359–1369. doi: 10.1016/s0042-6989(98)00007-8. [DOI] [PubMed] [Google Scholar]
- Von Schant ZM, Szel A, vanVee, Farber DB. Expression of soluble phototransduction-associated proteins in ground squirrel retina. Investigative Ophthalmology & Visual Science. 1994;35:3922–3930. [PubMed] [Google Scholar]
- Wassle Parallel processing in the mammalian retina. Nature Review Neuroscience. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Witkovsky Photoreceptor classes and transmission at the photoreceptor synapse in the retina of the clawed frog, Xenopus laevis. Microscopy Research and Technique. 2000;50:338–346. doi: 10.1002/1097-0029(20000901)50:5<338::AID-JEMT3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Stone S. Rod and cone inputs to bipolar and horizontal cells of the Xenopus retina. Vision Research. 1983;23:1251–1258. doi: 10.1016/0042-6989(83)90100-1. [DOI] [PubMed] [Google Scholar]
- Wu SM, Gao F, Maple BR. Functional architecture of synapses in the inner retina: Segregation of visual signals by stratification of bipolar cell axon terminals. Journal of Neuroscience. 2000;20:4462–4470. doi: 10.1523/JNEUROSCI.20-12-04462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Hazard ES, 3rd, Lockman DK, Crouch RK, Ma J. Molecular cloning of the salamander red and blue cone visual pigments. Molecular Vision. 1998;4:10. [PubMed] [Google Scholar]
- Yang JH, Maple B, Gao F, Maguire G, Wu SM. Postsynaptic responses of horizontal cells in the tiger salamander retina are mediated by AMPA-preferring receptors. Brain Research. 1998;797:125–134. doi: 10.1016/s0006-8993(98)00373-4. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Response sensitivity and voltage gain of the rod- and cone-horizontal cell synapses in dark- and light-adapted tiger salamander retina. Journal of Neurophysiology. 1996;76:3863–3874. doi: 10.1152/jn.1996.76.6.3863. [DOI] [PubMed] [Google Scholar]
- Yang XL, Wu SM. Response sensitivity and voltage gain of the rod- and cone-bipolar cell synapses in dark-adapted tiger salamander retina. Journal of Neurophysiology. 1997;78:2662–2673. doi: 10.1152/jn.1997.78.5.2662. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM. Co-localization of Shaker A-type K+ channel (Kv1.4) and AMPA-glutamate receptor (GluR4) immunoreactivities to dendrites of OFF-bipolar cells of goldfish retina. Journal of Neurocytology. 1999;28:63–73. doi: 10.1023/a:1007015801586. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Synaptic contacts between photoreceptors and on bipolar cells in the tiger salamander retina. Journal of Comparative Neurology. 2003;461:276–289. doi: 10.1002/cne.10704. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang Z, Wu SM. Immuocytochemical analysis of spatial organization of photoreceptors and amacrine and ganglion cells in the tiger salamander retina. Visual Neuroscience. 2004;21:157–166. doi: 10.1017/s0952523804042075. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang AJ, Wu SM. Immunocytochemical analysis of GABA-positive and calretinin-positive horizontal cells in the tiger salamander retina. Journal of Comparative Neurology. 2006a;499:432–441. doi: 10.1002/cne.21116. [DOI] [PubMed] [Google Scholar]
- Zhang AJ, Zhang J, Wu SM. Electrical coupling, receptive fields and relative rod/cone inputs of horizontal cells in the tiger salamander retina. Journal of Comparative Neurology. 2006b;499:422–431. doi: 10.1002/cne.21117. [DOI] [PubMed] [Google Scholar]


