Abstract
We investigated brain responses to matching versus nonmatching objects in working memory (WM) with a modified delayed match‐to‐sample task using event‐related potentials (ERPs). In addition, ERP correlates of new items (new matches/new nonmatches) and previously studied items (studied matches/studied nonmatches) were examined in the WM task. Half of the common visual objects were initially studied until 95% accuracy was attained and half were new. Each memory trial began with the presentation of a sample object followed by nine test objects. Participants indicated whether each test item was the same as the object held in mind (i.e., match) or a nonmatch. Compared to studied matches, new matches evoked activity that was 50 ms earlier and largest at frontal sites. In contrast, P3 activity associated with studied nonmatches was larger than for new nonmatches at mostly posterior sites, which parallels previously reported old–new ERP effects. The ERP source analysis further confirms that the cortical mechanisms underlying matching objects and rejecting irrelevant objects during the task are both temporally and spatially distinct. Moreover, our current findings suggest that prior learning affects brain responses to matching visual items during a WM task. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: ERPs, visual memory, matching, delayed match‐to‐sample, stimulus evaluation, common objects, LORETA
INTRODUCTION
Intentionally holding an item in mind for current use is an important function of working memory (WM), an active, short‐term memory mechanism [Baddeley, 1986; Baddeley and Hitch, 1974]. Psychophysiological studies have localized several neural structures critical to WM including the prefrontal cortex implicated in coordination processes characteristic of the central executive [D'Esposito, 2001; Goldman‐Rakic, 1996; Petrides, 1994; Postle, 2006; Ranganath, 2006; Wagner, 1999], posterior parietal cortex that participates in phonological coding and storage [Awh et al., 1996; Jonides et al., 1998; Postle and D'Esposito, 1999], thalamo‐cortical activity that is implicated in allocating attentional resources towards a specific cortical region [Birbaumer, 1990; LaBerge, 1997], inferior temporal (IT) cortex that is involved in comparing current visual images with internal representations [Miller and Desimone, 1994; Vogels and Orban, 1994; Zhang et al., 1995], and the premotor areas that are involved in the preparation of motor responses based on information held in mind [Petit et al., 1998].
Many psychophysiological studies of WM in primates and humans have used delayed match‐to‐sample tasks [Desimone, 1996; Jiang et al., 2000; Levy and Goldman‐Rakic, 2000]. Typically, a sample item is presented at the beginning of the trial and after a delay, is followed by one or more test items. One way researchers have been able to differentiate processing stages related to tracking matches and rejecting nonmatches is the use of event‐related potentials (ERPs). ERPs are voltage changes elicited by brain activity in response to specific events, which are recorded on subjects' scalps. Compared to indirect measures of neural activity (e.g. fMRI, which does not resolve cortical changes shorter than 1–2 s), EEG/ERPs provide a direct measure of electrical brain activity with a temporal resolution in the millisecond range.
One component that has been shown to index WM processes is the P3, a positive deflection occurring between 300 and 800 ms poststimulus. Although the P3 is considered a summation of several functionally independent neural mechanisms, two processes central to WM include a frontal P3a mechanism that is sensitive to novelty detection [Courchesne et al., 1975; Squires et al., 1975] and a posterior mechanism, P3b, with changes in amplitude reflecting the allocation of attentional resources [Polich, 1998], and the updating of environmental events in WM [Donchin and Coles, 1988]. P3b peak latency is generally considered an index of stimulus evaluation processes that are independent of brain activity related to motor response selection, preparation, and execution [Fournier et al., 2000; Kutas, 1977; Magliero et al., 1984; McCarthy and Donchin, 1981]. WM resources are utilized during the retrieval of explicit memories during task performance, and the presence of such explicit memories are also indexed by P3b activation.
A large body of (ERP) research has examined responses to studied or “old” items versus nonstudied or “new” items (for reviews see Friedman and Johnson, 2000; Paller, 2001]. Generally, ERP studies have indexed old–new effects by virtue of more positive going amplitude values for old than new items. Three separate components have been found to discriminate old from new items. One component, the early frontal old–new effect corresponding to a negative peak occurring around 400 ms, is smaller for old than new items and has been associated with familiarity processes that are a form of incidental learning [Curran, 2000; Johnson et al., 1998; Smith, 1993]. Recent studies, however, have indicated that this component is also sensitive to conceptual fluency between study and test phases [Jacoby, 1996; Toth, 1996; Wagner et al., 1997] and perceptual fluency derived from the lack of stimulus novelty between study and test [Schloerscheidt and Rugg, 2004; Tsivilis et al., 2001]. Thus, this early frontal old–new effect seems to be dependant on both semantic and perceptual similarity between encoding and retrieval.
A second component, termed the temporo‐parietal old–new effect, typically ranges from 400 to 800 ms and is often larger over the left than right hemisphere. The increased amplitude of this effect has been well supported as an index of recollection during the retrieval of old items [Düzel et al., 1997; Johnson et al., 1998; Smith, 1993]. Recollection reflects contextual memories of when and where an item was encountered and is sensitive to differences in levels of processing [Rugg et al., 2000] and attentional constraints [Curran, 2004].
The last known component, termed the late frontal effect, is maximal over the right central‐frontal scalp, and can range from 500 ms to as long as 2,000 ms post stimulus. Although, the cognitive function(s) reflected by this component is unclear, several studies have indicated that it may reflect retrieval processes relating to source memory [Swick and Knight, 1999; Wilding and Rugg, 1996].
The goal of the present study was to examine the neural mechanisms relating to WM performance of prior learned and new objects. Although studies examining old–new effects typically incorporated WM mechanisms during retrieval tasks, the majority of these studies did not examine how processes specifically related to WM were affected by prior learning. The present study examined the influence of prior learning on tracking and discrimination processes that are dependant on the WM status of a visual object. That is, the match or nonmatch status of an object evokes differential WM processes.
To examine psychophysiological differences in both WM for matching objects and prior learning, we adopted a delayed match‐to‐sample task previously used in monkey physiology [Miller and Desimone, 1994] and human brain imaging studies [Caggiano et al., 2006; Jiang et al., 2000]. For each trial, participants held a sample object in mind that was either previously studied or new and then determined whether each of the nine subsequent test objects matched the sample object. Half of matching and nonmatching objects were learned and half were new. This design allowed us to examine the neural mechanisms relating to (a) differences between responses to matches versus nonmatches, (b) differences relating to new versus studied objects, and (c) differences in searching for a new match versus a studied match among both new and studied nonmatches.
On the basis of previous research findings, we hypothesized that responses to matches would require more prefrontal WM resources, indexed by virtue of increased P3 activation. In addition, nonmatching objects correctly rejected as a nonmatch were hypothesized to evoke P3 activation from a mostly posterior source. Furthermore, we also hypothesized that new matching objects would result in a larger ERP response in frontal sites. Last, we hypothesized that prior studied objects would have larger and more positive activation than new objects based on previous reports of old–new ERP effects.
METHOD
Participants
Fourteen students (five male and nine female, M age = 22.3 years) from the University of Kentucky participated in the experiment and received monetary compensation. Inclusion criteria included normal or corrected 20/40 visual acuity, right hand dominance, between 18 and 28 years of age, and English as their first language.
Stimuli
Stimuli consisted of a set of 240 two‐dimensional pictures of common objects taken from Snodgrass and Vanderwart [1980]. Each picture was presented in white with a black background and within a rectangular area of ∼8.3 × 5.8 cm2. When appropriate, some pictures were also presented with a 6.5 mm green border. The picture set was divided into two goups with 60 pictures being initially studied by participants and 180 new pictures not previously studied.
Working Memory Task
The short‐term memory task consisted of 120 trials separated into 12 blocks of 10 trials each. Each trial began with the presentation of the sample picture (for 3,000 ms) that was distinguished by a green border (Fig. 1). A single tone presented at the onset of the sample picture further distinguished it from other pictures. The sample picture was followed by nine successive test pictures with a stimulus duration of 700 ms per picture. All test pictures were divided by an ISI of 1,100 ± 100 ms that contained a fixation cross. Each trial lasted ∼21 s.
Figure 1.
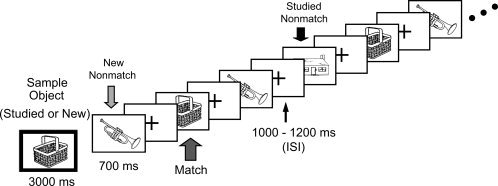
In this working memory task, a sample object was initially presented, followed by 9 successive test pictures (matching and nonmatching). Each WM trial lasted ∼21 s.
The 120 trials were divided into two groups with 60 trials utilizing a studied sample and 60 trials having a new sample. The order of studied and new sample trials was balanced in a pseudo‐random sequence that was consistent across subjects. Within trials, test pictures were classified into one of three groups: (a) matches (studied and new grouping depended on the sample trial type), (b) studied nonmatches, and (c) new nonmatches. On average, two nonmatching objects were presented for every matching object in a trial. Each of the studied pictures (n = 60) initially served as a studied match in one trial and served as a studied nonmatch in another trial. Of the 180 new objects, 60 objects served as new matches, and 120 objects served as new nonmatches. Thus, new objects, whether serving as a match or nonmatch, were not used in any subsequent trials.
The test portion of each trial contained a pseudo‐random presentation of matching and nonmatching objects. Matching objects were presented two, three, or four times, and nonmatching objects were each presented two, three, or four times making up a total of nine test objects per trial. This pseudo‐random presentation resulted in 180 events for each condition equaling 1,080 events total.
Procedure
The participants were instructed to study and memorize 60 pictures by performing a computerized naming task that lasted ∼10 min. Subjects also continued to study these pictures in paper form during the placement of an EEG cap, which lasted about 20 min. Subjects were told to relate the objects to personal experiences, and that they would be tested after placement of the EEG cap. A subsequent computer recognition test of the 60 studied objects interspersed among 60 nonstudied, new objects resulted in recognition accuracy scores no less than 96% (M = 98.2%).
Participants were told to hold the sample object in their mind and indicate whether each of the following nine test objects was the same or different from the sample picture by pressing one of two buttons using their right or left hand. Assignment of hands to indicate a match versus a nonmatch was counterbalanced across subjects. Subjects were also instructed to forget the previous sample object only when a new sample appeared. All participants performed 1 or 2 blocks of practice trials prior to data collection and the WM task lasted ∼60 min overall.
ERP Recordings
Electroencephalographic recordings were made from 62 scalp sites using Ag/AgCL electrodes embedded in an elastic cap at locations designed to provide fairly even coverage across the scalp. Two additional channels were used for monitoring horizontal and vertical eye movements. Trials with incorrect responses and trials contaminated by electro‐ocular artifacts were excluded from ERP analyses. A left mastoid reference electrode was used online and the reference was changed offline to the average of left and right mastoid recordings. Impedance was less than 5 KΩ. EEG signals were filtered with a band‐pass of 0.05–40 Hz and sampled at a rate of 500 Hz. Each averaging epoch lasted 1,000 ms, including 100 ms prior to stimulus onset.
Statistical Analyses
Behavioral effects were indexed using mean response times (RT) of correct responses and response accuracy data for each condition. ERPs were averaged for correct responses elicited by each condition recorded during the DMS task. Topographic maps were also examined, and visual inspection of these results from all 62 scalp locations indicated that the midline sites FPz, Fz, Cz Pz, and Oz provided a good index of neural activation for each condition.
On the basis of the visual inspection of effects for each condition, ERP mean amplitude data were initially gathered at time segments 200–350 and 350–500 ms (−100–0 ms set to 0 μV). Specific to match responses, ERP mean amplitude data were also gathered at time segments 200–300 and 300–400 ms. Differences between match and nonmatch responses could conceivably have been due to a repetition effect resulting from initially viewing the sample (matching) object at the beginning of each trial [for discussion see Jiang et al., 2000; Miller and Desimone, 1994]. Therefore, data were averaged from all presentations of an object within a trial.
Data for each behavioral measure were submitted to a two‐way repeated‐measures analysis of variance (ANOVA); with factors relating to match trial type (studied match, new match) and test object type (match, studied nonmatch, new nonmatch). A global four‐way repeated‐measures ANOVA, i.e., match trial type (studied match, new match), test object type (matches, studied nonmatches, new nonmatches), electrode site (Fpz, Fz, Cz, Pz, Oz), and time interval (200–350, 350–500 ms), was conducted on mean amplitude data. Subsequent planned comparisons focusing on match trial type (studied match, new match), electrode site (Fpz, Fz, Cz, Pz, Oz), and time interval (200–300, 300–400 ms) were also conducted.
All ANOVAs had a level of significance set to 0.05 and were supplemented with Bonferroni pairwise comparisons or simple main effects comparisons when appropriate. Greenhouse‐Geisser corrections were reported with all effects having two or more degrees of freedom in the numerator.
An intracranial source analysis was calculated for each time point between 200 and 500 ms, as well as at the time point with the maximal signal strength as estimated by mean global field power. Analyses were conducted for all difference waves (i.e., matches minus nonmatches, new matches minus studied matches, and new nonmatches minus studied nonmatches). We used the LORETA method (low resolution electromagnetic tomography, via Curry V5.0), a new method for localizing electrical activity in the brain [Anderer et al., 1998], which uses a Laplacian model term. The Laplacian measures the second derivative of source strengths. Current density reconstructions (CDRs) assume simultaneous activity at a large number of possible source locations. Prior results have shown that LORETA produces blurred but accurate localizations of point sources [Guo et al., 2006; Pascual‐Marqui et al., 2002]. The procedure used a realistic volume conductor model derived using a boundary element method with three layers [skin (10 mm), skull (9 mm), and brain (7 mm), with conductivities of 0.3300, 0.0042, and 0.3300, respectively].
RESULTS
Behavioral Measures
Response time and response accuracy data for each match and nonmatch condition are summarized in Table I. For response time, a significant main effect of test object type, F(2, 26) = 9.986, P < 0.01, indicated that responses to matches were slower than responses to both studied and new nonmatches. For response accuracy, a significant main effect of test object type, F(2, 26) = 40.775, P < 0.001, was also found. Responses to matches were less accurate than responses to both studied and new nonmatches.
Table I.
Mean percentage of correct responses and response times
| Targets | Studied distracters | New distracters | |
|---|---|---|---|
| Response times (msec) | |||
| Studied sample targets | 486 (14) | 463 (12) | 465 (11) |
| New sample targets | 483 (15) | 460 (11) | 464 (11) |
| Accuracy (%) | |||
| Studied sample targets | 90.7 (1.2) | 97.3 (0.5) | 98.0 (0.5) |
| New sample targets | 91.2 (1.5) | 97.5 (0.5) | 98.3 (0.3) |
Standard error is given in parentheses.
ERP Measures
Corresponding ERPs averaged across all 14 subjects are shown in Figure 2 for match responses and Figure 3 for nonmatch responses. Visual inspection of ERP responses among condition types showed that beginning about 200 ms after stimulus onset, ERPs for match responses were more positive than those for nonmatching objects. Also, ERPs, recorded at parietal and occipital sites were visually more positive when a studied nonmatch was presented compared to a new nonmatch. Beginning about 550 ms, condition types were generally superposed.
Figure 2.
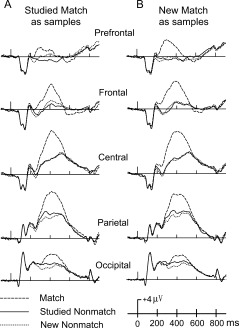
ERPs from the two nonmatch object types (i.e., new nonmatches, studied nonmatches) within a trial along with studied matches (A) or new matches (B) between trials.
Figure 3.
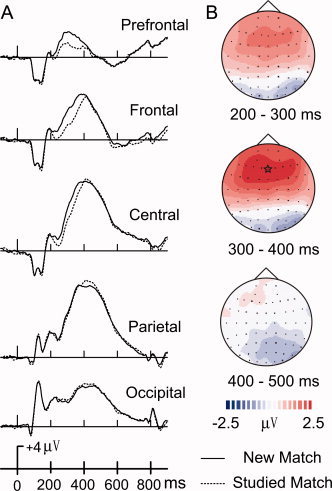
(A) ERP comparisons between new and studied matches across five midline scalp locations (FPz, Fz, Cz, Pz, Oz). Topographic maps (B) illustrate ERP difference waves computed by subtracting ERPs to studied matches from ERPs to new matches across three intervals (200–300, 300–400). The largest activation difference is indicated by a star.
Visually comparing ERPs evoked by new and studied matches, new match ERPs elicited more positive amplitudes than studied matches at prefrontal sites 200–550 ms after stimuli onset (Fig. 3). ERPs of new and studied matches generally peaked about 400 ms at the central site. Figure 4 illustrates ERPs comparisons of new and studied nonmatches. Studied nonmatches elicited more positive amplitudes than new nonmatches at posterior sites 250–600 ms after stimuli onset. ERPs of new and studied nonmatches generally peaked about 500 ms at the central site.
Figure 4.
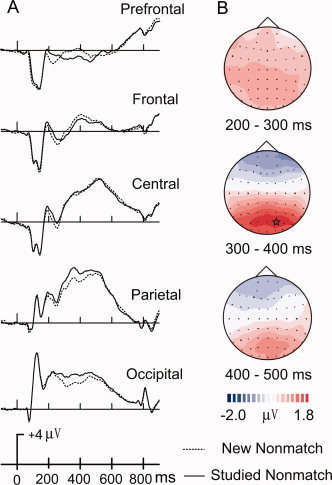
(A) ERP comparisons between new nonmatches and studied nonmatches across five midline scalp locations (FPz, Fz, Cz, Pz, Oz). Topographic maps (B) illustrate ERP difference waves computed by subtracting ERPs to studied nonmatches from ERPs to new nonmatches across three intervals (200–300, 300–400). The largest activation difference is indicated by a star.
A main effect of test object type, F(2,26) = 149.34, P < 0.001, was found (Fig. 2). Planned comparisons indicated that matches were more positive than both studied and new nonmatches (P = 0.001). A significant interaction between test object type and electrode, F(8,104) = 34.43, P < 0.001, was found. Planned comparisons indicated that studied nonmatches were more positive than new nonmatches at sites Pz and Oz (P < 0.001). A more focused comparison between studied nonmatches and new nonmatches is shown in Figure 4. The topography map of the difference wave showed an evident old–new parietal effect. Also, a significant interaction between test object type and interval, F(2,26) = 20.82, P < 0.001, indicated that matching objects had more positive activation at the 350–500 ms interval than during the 200–350 ms interval. Last, a significant four‐way interaction among match trial type, test object type, interval, and electrode, F(8,104) = 4.516, P = 0.007, indicated that ERP differences were sensitive to the interactions between all variables. Pair‐wise comparisons revealed no significant main or interaction effects for nonmatch types in the studied match condition (Fig. 2A) versus nonmatches in the new match condition (Fig. 2B), P > 0.05. Therefore, further comparisons focused on responses to studied versus new matching objects.
Planned Comparisons Between Studied and New Matches
To better capture the ERP effects for matching objects, the time intervals were shortened from 200–350 and 350–500 ms to 200–300 and 300–400 ms, respectively. There were significant main effects of matching objects, F(1,13) = 23.04, P < 0.001, electrode site, F(4,52) = 13.26, P < 0.001, and time interval, F(1,13) = 45.25, P < 0.001 (Fig. 3). Overall, larger positive amplitudes were found for new matches, frontal and central electrode sites, and for the 300–400 ms time interval. A significant 3‐way interaction indicated that ERP differences between studied matches and new matches varied with electrodes and intervals, F(4,52) = 7.05, P = 0.001. Subsequently, we analyzed the relationship between studied matches and new matches for each electrode and each interval separately.
Between 200 and 300 ms, ERPs of studied matches and new matches were significantly different, F(1,13) = 25.63, P < 0.001, with new matching objects having larger positive amplitudes (Fig. 3A). Planned comparisons between match type and electrode, F(4,52) = 13.86, P < 0.001, indicated that new matches had higher mean amplitude values than studied matches at Fpz, Fz, and Cz (P ≤ 0.001). The ERP differences between studied matches and new matches were not significant at Pz and Oz. Between 300 and 400 ms, ERP differences between studied matches and new matches were identical to the 200–300 ms interval. Figure 3 illustrates that the more enhanced frontal activity for new matches between 200 and 400 ms is due to the P3 incline occurring 50 ms earlier for new matches than studied matches.
Comparisons of WM (Studied) and WM (New)
Figure 5 illustrates that both WM types were focused centrally, but WM processes for new objects (WMN, subtraction of new match minus new nonmatch activation) had earlier latencies than WM processes for studied objects (WMS, subtraction of studied match minus studied nonmatch activation) by about 50 ms. ERP mean amplitudes at time intervals 200–300 and 300–400 ms were used in significance testing. A significant main effect of WM, F(1,13) = 23.77, P < 0.001, found that WMN was more positive than WMS (Fig. 5A). A three‐way interaction among WM, electrode, and interval conditions, F(4,52) = 8.96, P = 0.001, was also significant. Planned pair‐wise comparisons revealed that between 200 and 300 ms, WMN was more positive than WMS at three midline sites (Fpz: P = 0.008; Fz: P < 0.001; Cz: P < 0.001). No difference was found for WM at Pz and Oz. Similar results were found for the 300–400 ms interval except that WMN was more positive than WMS at all five electrode sites (P ≤ 0.006). The difference between the two WM conditions focused on the frontal, central, and parietal sites from 200 to 400 ms (Fig. 6). They were overlapped, however, from 100 to 200 ms and from 400 to 500 ms.
Figure 5.
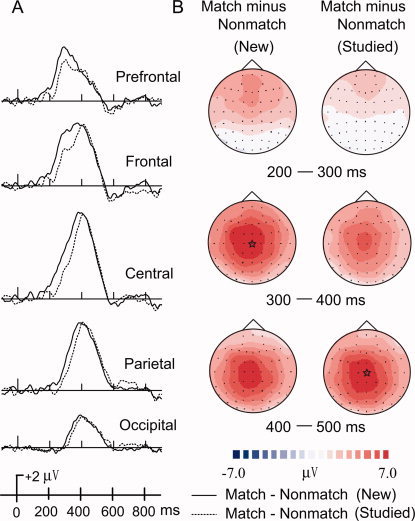
ERPs comparisons between WM for new matches and WM for studied matches are shown from (A) five midline scalp locations (Fpz, Fz, Cz, Pz, Oz). WM difference waves were computed by subtracting ERPs to nonmatches from matches for both old and new objects. Topographic maps (B) illustrate WM difference waves across three intervals (200–300, 300–400). The largest activation differences are indicated by stars.
Figure 6.
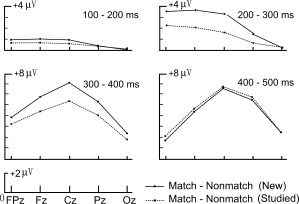
Mean ERP amplitudes for difference waves relating to WM for new matches (match minus nonmatch) and WM for studied matches (match minus nonmatch) from five midline scalp locations (Fpz, Fz, Cz, Pz, Oz) and four intervals (100–200, 200–300, 300–400, 400–500 ms).
Comparisons Between Conditions With Source Localization
Results from intracranial source analyses calculated using the LORETA method are shown in Figure 7. These results correspond to the interval from 200 to 500 ms in the scalp topographic results shown in Figures 3, 4, 5. In each case, source analyses conducted showed similar patterns across this time interval and the time points for this interval provide representative findings.
Figure 7.
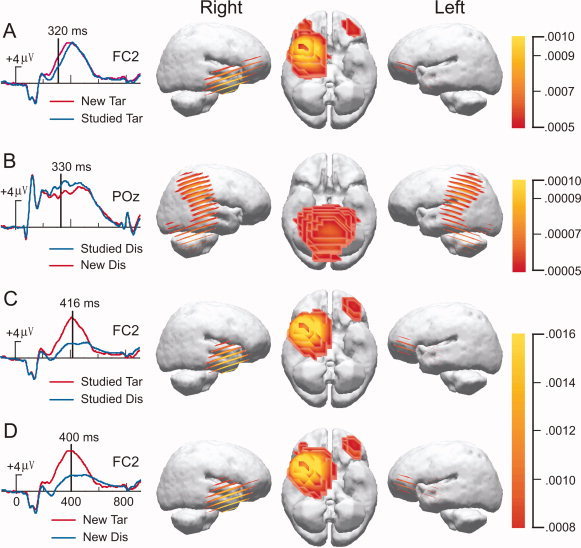
Results from intracranial source analysis for ERP comparisons. Low‐resolution current density reconstructions based on the LORETA model are shown via a color scale for current density reconstructions (CDRs) as computed at the designated time point superimposed on left and right hemispheres. The left column shows the electrode location with the largest activity.
The two different WM status effects (Fig. 7C,D) were similar with respect to the right prefrontal and right frontal source found with this method. Similar source localizations were also found in the case of new matches minus studied matches (Fig. 7A), whereas cortical differences of studied minus new nonmatches reflected strong bilateral activation at temporal and parietal cortices, including ventral temporal object areas (Fig. 7B). Interestingly, the frontal and bilateral ventral temporal sources provide important clues for direct comparisons with fMRI findings [Jiang et al., 2000], but further research is needed to substantiate these intracranial source estimations.
DISCUSSION
Using a WM task that involved matching visible objects to an object held in mind, the current study revealed that brain potentials indexed processes associated with both WM and prior learning. WM for identifying matching objects had greater positive activation than for nonmatch responses at frontal sites. Two separate old–new effects also differentiated matches from nonmatches. Responses to studied matches had later and smaller P3 activation than new matches at frontal sites. Studied nonmatches, however, had greater P3 activation than new nonmatches at posterior sites, a classic old–new effect [Paller, 2001]. Interplay between WM and prior learning was further revealed with differences in WM status for new items (new matches versus new nonmatches) having larger effects on P3 activation than differences in the WM status of old items (studied matches versus studied nonmatches). This finding shows that the brain responds differently to matches and nonmatches during a delayed match‐to‐sample task depending on whether new or old objects are encountered.
Behaviorally, participants' responses to matching items were consistently slower and less accurate than those to nonmatches. In a similar WM task using studied faces, Caggiano et al. [2006] reported that young and older subjects responded to matching faces slower than to nonmatching faces, which is consistent with the current behavioral results. Between 200 and 400 ms, ERPs also revealed an obvious separation between matches and nonmatches. Matches, irrespective of being new or old, elucidated more P3 activation than nonmatches.
To correctly perform the matching task, participants had to perform three general operations [Desimone, 1996]. First the subject had to attend and perceive the sample stimulus. Research has shown that attention has an effect on early visual processing in the occipital and temporal lobes as early as 100 ms following the presentation of the eliciting stimulus [Hopfinger et al., 2001]. Second, subjects had to hold the sample in memory for the length of the trial. Last, subjects had to evaluate the test stimulus and make a decision about whether it matched the sample in memory, which is often indexed by P3 activation.
In our WM task, matches were the task‐relevant objects, which were matched to the memory trace held in mind. Matching objects, therefore, were expected to demand more attentional resources than nonmatching objects because participants had to match both semantic and perceptual features to make a correct response. Task relevance and attention has long been associated with P3 activation while ignored or nonattentive items typically have reduced P3 amplitude [Kok, 2001; Pritchard, 1981]. Current behavioral and ERP results are consistent with this interpretation that correctly recognizing an item as matching the one held in memory requires more attentional resources than nonmatching items.
A primary aim of this study was to examine differences in tracking a studied match (e.g., an old friend) versus a new match (e.g., a new friend) among nonmatches. We observed that ERPs of new matches had larger positive amplitudes than for studied matches between 200 and 400 ms poststimulus (Fig. 2).
For matching objects only, ERP activation relating to the P3 incline occurred 50 ms earlier for new than studied matches at frontal sites (Fig. 3). While it has long been known that prior learning affects WM, the present results extend our understanding by revealing that matching a visual object to the one held in mind is accompanied by an evaluation of an objects' old–new status, even though this evaluation of old–new status is incidental to performing the task.
The differential activation pattern for new and studied matches may be due to distinct inputs that the prefrontal cortex receives from the ventral temporal and medial temporal regions. Prefrontal activation is known to be important for stimulus evaluation processes during WM [Desimone, 1996; Goldman‐Rakic, 1996; Levy and Goldman‐Rakic, 2000], and is confirmed by our source activation patterns over prefrontal areas (Fig. 7). Consistent with the current source results, the prefrontal cortex receives input from ventral temporal regions (e.g. fusiform face and object areas) which are involved in perceptual processes [Haxby et al., 2001; Kanwisher et al., 1997] and medial temporal areas which are important for recognition memory. Stimulus evaluation required matching the test object's perceptual/physical features with that held in mind but did not necessarily require evaluation of its old–new status. Our results show, however, that matching an item involved both brain structures involved in perceptual processing and structures involved old–new processing.
On the basis of these findings, we propose that the 50 ms delay for studied matches reflects activation of the medial temporal lobe for checking its old–new status. This hypothesis leads to the prediction that brain aging or dementia will result in the loss of this timing difference in processing old and new matching items. In support of this hypothesis, recent pilot data of healthy older adults who performed a matching task showed no ERP activation differences in processing new and old matching items [Lawson et al., 2007].
A distinct ERP old–new effect was also found for responses to nonmatching objects. Studied nonmatches elicited more positive ERPs than new nonmatches at posterior sites between 200 and 550 ms poststimulus (Fig. 4). Our old–new effect for nonmatching objects is most consistent with a large body of research, which collectively indicates that such a temporo‐parietal old–new effect reflects recollection processes characteristic of intentionally studied items [Paller, 2001].
To determine whether WM processes in the brain were affected differentially to a novel or familiar environment, we examined ERP difference waves for WM of new and old objects. Each difference wave was determined by subtracting the match response ERP from the nonmatch response ERP for new and old objects, respectively. Our results revealed that brain responses reflecting changes in the WM status of an object differ with respect to both amplitude and time course. WM processes relating to new objects began about 50 ms earlier and had more divergent P3 activation than WM processes to old objects, with this amplitude difference occurring during the P3 incline (i.e., 200–400 ms) (Fig. 5). Utilizing a similar WM task with familiar faces, Jiang et al. [2000] found that enhanced fMRI responses occurred at frontal/insular areas for matches over nonmatches. They suggested that such frontal activation might be indicative of WM processes, since this activation reflected the memory status of tracking or holding matches in mind. The scalp distribution of the current WM effect (Fig. 6) and source localization results also indicate a frontal source for matching objects held in mind (similar to a novelty P3) during this WM task. Activation of nonmatch objects, in contrast, reflected the more typical P3b activation occurring at parietal and IT areas [Jiang et al., 2000].
Previous studies have extensively examined studied matches and nonmatches extensively using the classical oddball paradigm [Kok, 2001]. Subjects responded to task‐relevant infrequent events (oddball matches), eliciting the classic P3, or P3b, over parietal sites. In contrast, nonmatches during an oddball paradigm evoked P3 components at more frontal areas, contributing to the novelty P3. Thus, the novelty P3 and P3b components in odd‐ball tasks may indicate the involvement of distinct attentional subsystems that reflect match versus nonmatch processing [Bledowski et al., 2004]. Although our current results show a similar frontal‐match and posterior‐nonmatch pattern of activation, matches and nonmatches in the present study reflected differences in WM status as opposed to attention. Unlike most oddball paradigms, matches and nonmatches in the present study were visually identical and the same object could serve as a match in one trial and a nonmatch in another. Thus, the memory status of an object changed from trial to trial, emphasizing WM processes necessary for task performance.
While the differences between matches and nonmatches reflects their respective WM status, P3 activation can be evoked by many WM subprocesses and changes in amplitude can be influenced by many factors including task relevance, novelty, attention capacity, and memory load [Johnson, 1986; Kok, 2001; Rees et al., 1997; Spinks et al., 2004]. Also, increased P3 amplitude indicates correct detections and rejections in decision making and confidence level, and decreased P3 amplitude indicates difficulty of discrimination [Andreassi, 2000]. Differences in prior learning between matches and nonmatches could be affected by one or many of these WM processes. Future research is needed to disentangle these cognitive mechanisms, especially in relation to novelty and familiarity processes as they have been shown to readily affect P3 activation [Paller, 2001].
Although the present old–new effects may be best understood in relation to an object's WM status, a more specific interpretation must be made with caution. Our WM task differed from prior studies examining old–new effects in two important ways. First, our delayed matching‐to‐sample task required participants to hold an object in memory while responding to several test objects. To our knowledge, prior studies that used a matching task only required one comparison to each object held in mind [Talsma et al., 2001; Zhang et al., 1995]. This within trial difference could account for discrepancies between past studies and the present findings, since our task required the object to be held in mind longer and allowed for within‐trial interference, neither of which have been adequately studied in relation to old–new effects.
A second factor that complicates a more specific interpretation of the differing old–new effects is that all objects used in our study were common objects, which could have lessened the ability to detect effects of prior learning, especially in relation to familiarity. Future research should examine how semantic and episodic memories as well as distinctions in explicit and implicit learning of objects affect such interactions between intentional learning and WM processes.
CONCLUSIONS
Our results demonstrated that prior learning affects both the tracking of visual matches and rejecting nonmatches during a WM task. Matching new objects evoked faster and more frontal responses than that of studied objects. Given the frontal lobes' differential treatment of new from old objects held in mind, future research should further examine this interplay between WM and prior learning.
Supporting information
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat .
Part of the results from this study was presented at the Annual meeting for Human Brain Mapping conference 2005.
Contributor Information
Chunyan Guo, Email: guocy@mail.cnu.edu.cn.
Yang Jiang, Email: yjiang@uky.edu.
REFERENCES
- Anderer P,Pascual‐Marqui RD,Semlitsch HV,Saletu B ( 1998): Differential effects of normal aging on sources of standard N1, target N1 and target P300 auditory event‐related brain potentials revealed by low resolution electromagnetic tomography (LORETA). Electroencephalogr Clin Neurophysiol 108: 160–174. [DOI] [PubMed] [Google Scholar]
- Andreassi JL ( 2000): Event‐related potentials and behavior. II. Mental, sensory, attentional, and perceptual activities In: Psychophysiology, 4th ed. New Jersey: Lawrence Erlbaum. [Google Scholar]
- Awh E,Jonides J,Smith EE,Schumacher EH,Koeppe RA,Katz S ( 1996): Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychol Sci 7: 25–31. [Google Scholar]
- Baddeley AD ( 1986): Working Memory. Oxford: Clarendon Press. [Google Scholar]
- Baddeley AD,Hitch G ( 1974): Working memory In: Bower GA, editor. Recent Advances in Learning and Motivation, Vol. 8 New York: Academic Press; pp 47–90. [Google Scholar]
- Birbaumer N,Eldert T,Canavan AGM,Rockstroh B ( 1990): Slow potentials of the cerebral cortex and behavior. Physiol Rev 70: 1–41. [DOI] [PubMed] [Google Scholar]
- Bledowski C,Prvulovic D,Hoechstetter K,Scherg M,Wibral M,Goebel R,Linden DE ( 2004): Localizing P300 generators in visual target and distractor processing: a combined event‐related potential and functional magnetic resonance imaging study. J Neurosci 24: 9353–9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiano DM,Jiang Y,Parasuraman R ( 2006): Aging and repetition priming for targets and distracters in a working memory task. Aging Neuropsychol Cogn 13: 552–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E,Hillyard SA,Galambos R ( 1975): Stimulus novelty, task relevance, and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol 39: 131–143. [DOI] [PubMed] [Google Scholar]
- Curran T ( 2000): Brain potentials of recollection and familiarity. Mem Cognit 28: 923–938. [DOI] [PubMed] [Google Scholar]
- Curran T ( 2004): Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia 42: 1088–1106. [DOI] [PubMed] [Google Scholar]
- Desimone R ( 1996): Neural mechanisms for visual memory and their role in attention. Proc Natl Acal Sci USA 93: 13494–13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E,Coles MGH ( 1988): Is the P300 component a manifestation of context updating? Behav Brain Sci 11: 357–427. [Google Scholar]
- Düzel E,Yonelinas AP,Mangun GR,Heinze HJ,Tulving E ( 1997): Event‐related brain potential correlates of two states of conscious awareness in memory. Proc Natl Acad Sci USA 94: 5973–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M ( 2001): Functional neuroimaging of working memory In: Cabeza R,Kingstone A, editors. Handbook of Functional Neuroimaging of Cognition. Cambridge: MIT Press; pp 293–327. [Google Scholar]
- Fournier LR,Scheffers MK,Coles MGH,Adamson A,Abad EV ( 2000): When complexity helps: An electrophysiological analysis of multiple feature benefits in object perception. Acta Psychologica 104: 119–142. [DOI] [PubMed] [Google Scholar]
- Friedman D,Johnson R Jr ( 2000): Event‐related potential (ERP) studies of memory encoding and retrieval: A selective review. Microsc Res Tech 51: 6–28. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS ( 1996): The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos Trans Res Soc Lond Biol Sci 351: 1445–1453. [DOI] [PubMed] [Google Scholar]
- Guo C,Li DW,Paller KA ( 2006): Distinguishing source memory and item memory: Brain potentials at encoding and retrieval. Brain Res 1118: 142–154. [DOI] [PubMed] [Google Scholar]
- Haxby JV,Gobbini MI,Furey ML Ishai A,Schouten JL,Pietrini P ( 2001): Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293: 2425–2430. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB,Woldorff MG,Fletcher EM,Mangun GR ( 2001): Dissociating top‐down attentional control from selective perception and action. Neuropsychologia 39: 1277–1291. [DOI] [PubMed] [Google Scholar]
- Jacoby LL ( 1996): Dissociating automatic and consciously controlled effects of study/test compatibility. J Mem Lang 35: 35–52. [Google Scholar]
- Jiang Y,Haxby JV,Martin A,Ungerleider LG,Parasuraman R ( 2000): Complementary neural mechanisms for tracking items in human working memory. Science 287: 643–646. [DOI] [PubMed] [Google Scholar]
- Johnson R Jr ( 1986): A triarchic model of P300 amplitude. Psychophysiology 23: 367–384. [DOI] [PubMed] [Google Scholar]
- Johnson R Jr,Kreiter K,Russo B,Zhu J ( 1998): A spatio‐temporal analysis of recognition‐related event‐related brain potentials. Int J Psychophysiol 29: 83–104. [DOI] [PubMed] [Google Scholar]
- Jonides J,Schumacher EH,Smith EE,Koeppe RA,Awh E,Reuter‐Lorenz PA,Marshuetz C,Willis CR ( 1998): The role of parietal cortex in verbal working memory. J Neurosci 18: 5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N,McDermott J,Chun MM ( 1997): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok A ( 2001): On the utility of P300 amplitude as a measure of processing capacity. Psychophysiology 38: 557–577. [DOI] [PubMed] [Google Scholar]
- Kutas M,McCarthy G,Donchin E ( 1977): Augmenting mental chronometry: The P300 as a measure of stimulus evaluation time. Science 197: 792–795. [DOI] [PubMed] [Google Scholar]
- LaBerge D ( 1997): Attention, awareness, and the triangular circuit. Concious Cogn 6: 149–181. [PubMed] [Google Scholar]
- Lawson AL,Guo C,Jiang Y ( 2007): Age effects on brain activity during repetition priming of targets and distracters. Neuropsychologia 45: 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R,Goldman‐Rakic PS ( 2000): Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res 133: 23–32. [DOI] [PubMed] [Google Scholar]
- Magliero A,Bashore TR,Coles MG.H,Donchin E ( 1984): On the dependence of P300 latency on stimulus evaluation processes. Psychophysiology 21: 171–186. [DOI] [PubMed] [Google Scholar]
- McCarthy G,Donchin E ( 1981): A metric for thought: A comparison of P300 latency and reaction time. Science 211: 77–80. [DOI] [PubMed] [Google Scholar]
- Miller EK,Desimone R ( 1994): Parallel neuronal mechanisms for short‐term memory. Science 263: 520–523. [DOI] [PubMed] [Google Scholar]
- Paller KA ( 2001): Neurocognitive foundations of human memory. Psychol Learn Motiv: Adv Res Theory 40: 121–145. [Google Scholar]
- Pascual‐Marqui RD,Esslen M,Kochi K,Lehmann D ( 2002): Functional imaging with low resolution brain electromagnetic tomography (LORETA): Review, new comparisons, and new validation. Jpn J Clin Neurophysiol 30: 81–94. [PubMed] [Google Scholar]
- Petit L,Courtney SM,Ungerleider LG,Haxby JV ( 1998): Sustained activity in the medial wall during working memory delays. J Neurosci 18: 9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M ( 1994): Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in nonhuman primates In: Boller F,Grafman J, editors. Handbook of Neuropsychology. Amsterdam: Elsevier; pp 59–82. [Google Scholar]
- Polich J ( 1998): P300 clinical utility and control of variability. J Clin Neurophysiol 15: 14–33. [DOI] [PubMed] [Google Scholar]
- Postle BR ( 2006): Working memory as an emergent property of the mind and brain. Neurosci 139: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR,D'Esposito M ( 1999): “What”‐then‐“Where” in visual working memory: an event‐related fMRI study. J Cogn Neurosci 11: 585–597. [DOI] [PubMed] [Google Scholar]
- Pritchard WS ( 1981): Psychophysiology of P300. Psychol Bull 89: 506–540. [PubMed] [Google Scholar]
- Ranganath C ( 2006): Working memory for visual objects: Complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neurosci 139: 277–289. [DOI] [PubMed] [Google Scholar]
- Rees G,Frith CD,Lavie N ( 1997): Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science 278: 1616–1618. [DOI] [PubMed] [Google Scholar]
- Rugg MD,Allen K,Birch CS ( 2000): Electrophysiological evidence for the modulation of retrieval orientation by depth of study processing. J Cogn Neurosci 12: 664–678. [DOI] [PubMed] [Google Scholar]
- Schloerscheidt AM,Rugg MD ( 2004): The impact of change in stimulus format on the electrophysiological indices of recognition. Neuropsychologia 42: 451–466. [DOI] [PubMed] [Google Scholar]
- Smith ME ( 1993): Neurophysiological manifestations of recollective experience during recognition memory judgments. J Cogn Neurosci 5: 1–13. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG,Vanderwart M ( 1980): A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol 6: 174–215. [DOI] [PubMed] [Google Scholar]
- Spinks JA,Zhang JX,Fox PT,Gao JH,Tan LH ( 2004): More workload on the central executive of working memory, less attention capture by novel visual distractors: Evidence from an fMRI study. Neuroimage 23: 517–524. [DOI] [PubMed] [Google Scholar]
- Squires NK,Squires KC,Hillyard SA ( 1975): Two varieties of long‐latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol 38: 387–401. [DOI] [PubMed] [Google Scholar]
- Swick D,Knight RT ( 1999): Contributions of prefrontal cortex to recognition memory: Electrophysiological and behavioral evidence. Neuropsychology 13: 155–170. [DOI] [PubMed] [Google Scholar]
- Talsma D,Wijers AA,Klaver P,Mulder G ( 2001): Working memory processes show different degrees of lateralization: Evidence from event‐related potentials. Psychophysiology 38: 425–439. [PubMed] [Google Scholar]
- Toth JP ( 1996): Conceptual automaticity in recognition memory: Levels‐of‐processing effects on familiarity. Canadian J Exp Psychol 50: 123–138. [DOI] [PubMed] [Google Scholar]
- Tsivilis D,Otten LJ,Rugg MD ( 2001): Context effects on the neural correlates of recognition memory: An electrophysiological study. Neuron 31: 497–505. [DOI] [PubMed] [Google Scholar]
- Vogels R,Orban GA ( 1994): Activity of inferior temporal neurons during orientation discrimination with successively presented gratings. J Neurophysiol 71: 1428–1452. [DOI] [PubMed] [Google Scholar]
- Wagner AD ( 1999): Working memory contributions to human learning and remembering. Neuron 22: 19–22. [DOI] [PubMed] [Google Scholar]
- Wagner AD,Gabrieli JDE,Verfaellie M ( 1997): Dissociation between familiarity processes in explicit recognition and implicit perceptual memory. J Exp Psychol Learn Mem Cogn 23: 305–323. [DOI] [PubMed] [Google Scholar]
- Wilding EL,Rugg MD ( 1996): An event‐related potential study of recognition memory with and without retrieval of source. Brain 119: 889–905. [DOI] [PubMed] [Google Scholar]
- Zhang XL,Begleiter H,Porjesz B,Wang W,Litke A ( 1995): Event related potentials during object recognition tasks. Brain Res Bull 38: 531–538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat .


