Blood flow to the brain is highly regulated through an array of interacting cells and molecular signals, all impinging on the vasculature. Although many mechanisms influence cerebrovascular resistance and thus cerebral blood flow,1 two of the most powerful regulators are molecular oxygen and carbon dioxide.1,2 The impact of sustained hypoxia (decreases in arterial PO2) and hypercapnia (increases in arterial PCO2) on cerebral blood flow has been studied widely in animal models and in people.2 Both hypoxia and hypercapnia can occur as a result of environmental factors but also occur under some pathophysiological conditions. For example, periodic hypoxia and hypercapnia are both key elements of sleep-disordered breathing, a group of abnormalities which include obstructive sleep apnea.3 Obstructive sleep apnea is an increasingly prevalent condition with a complex pathophysiology that encompasses activation of the sympathetic nervous system, vascular abnormalities, as well as disturbed sleep patterns.3 Obstructive sleep apnea is often associated with hypertension and is known to increase the risk for cerebrovascular disease, stroke, and cognitive decline.4,5
Because intermittent hypoxia is one element of obstructive sleep apnea and is relatively easy to produce experimentally, many investigators have studied effects of this type of hypoxia using animal models. While intermittent hypoxia does not mimic the entire array of changes that occur, there is evidence that brief repetitive periods of hypoxia contribute to the overall pathophysiology of obstructive sleep apnea.6 Previous studies of the effects of intermittent hypoxia have described diverse vascular effects that include activation of oxidant- and immune-related pathways, endothelial dysfunction, vascular hypertrophy, and the progression of atherosclerosis.3,6 Although obstructive sleep apnea is a risk factor for stroke and cognitive decline,4,5 relatively little is known regarding its effects on the cerebral circulation or the mechanisms involved. Previous studies have described changes in myogenic reactivity and endothelial function in isolated cerebral arteries in models of intermittent hypoxia.7,8
In this issue of Hypertension, Capone et al9 examined the hypothesis that intermittent hypoxia impairs the control of brain perfusion and that NADPH oxidase and endothelin-1 (ET-1) play key roles in producing such changes. Their approach included the study of young male mice made hypoxic intermittently by changing the inspired PO2 from ~147 to 70 mmHg every 90 seconds during their normal sleep time. In addition to quantification of changes in local cerebral blood flow, complementary methodology included measurements of superoxide levels, components of the endothelin system, and arterial blood pressure. Using this model, 14 days of intermittent hypoxia impaired cerebrovascular responses to endothelium-dependent agonists (both NO-dependent and NO-independent) and whisker stimulation. The latter involves activation of the somatosensory cortex and is a common model to examine neurovascular coupling (or functional hyperemia) in this region of the brain. These changes occurred in the absence of a significant increase in arterial pressure (in awake or anesthesized mice). A longer exposure to intermittent hypoxia (35 days) produced mild hypertension and tended to further reduce endothelium-dependent vasodilation and neurovascular coupling. Because impairment of regulation of cerebral blood flow was seen prior to increases in arterial pressure, the data suggest intermittent hypoxia produces cerebrovascular changes independent of hypertension.
Focusing on the longer duration of intermittent hypoxia, the authors next addressed potential mechanisms involved. Oxidative stress contributes to vascular abnormalities in many disease models including models of hypertension and intermittent hypoxia.3,6,10 Following intermittent hypoxia, superoxide levels were increased and vasodilator responses to acetylcholine and whisker stimulation were restored to normal by a scavenger of superoxide, an inhibitor of NADPH oxidase, or genetic deficiency in Nox2. Nox2 is the enzymatic component of one isoform of NADPH oxidase, a key source of reactive oxygen species in the vasculature.10
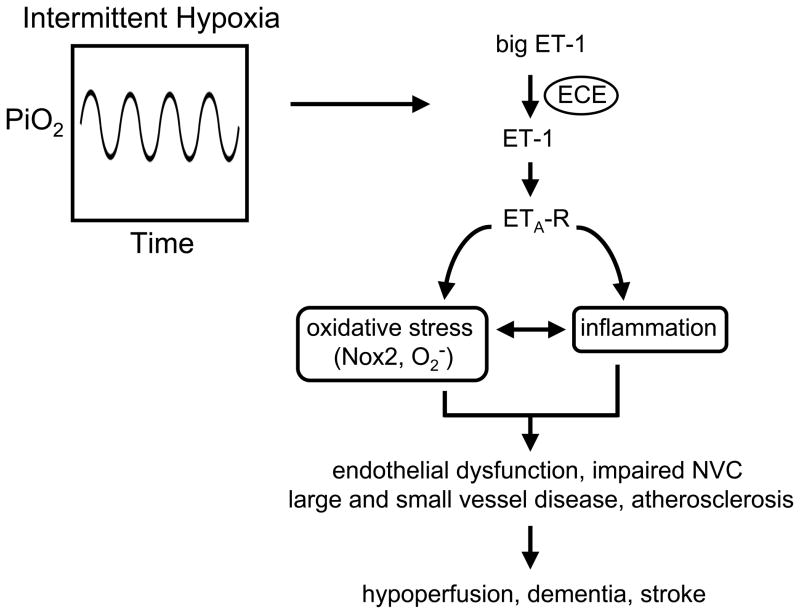
The endothelin system, particularly endothelin-1 (ET-1), has been previously implicated in vascular disease and hypertension as well as cardiovascular changes during intermittent hypoxia.3,11,12 Primarily via activation of ETA receptors, ET-1 is a powerful vasoconstrictor but also has pro-inflammatory and pro-oxidant effects in vascular cells,11 all features seen with intermittent hypoxia.3,6 In the current experiments, intermittent hypoxia increased local expression of endothelin-converting enzyme and ETA receptors, as well as producing remarkable increases in the levels of perivascular ET-1 (Figure). These changes were functionally important because an inhibitor of ETA receptors reduced superoxide and restored vascular responses (vasodilation to acetylcholine and whisker stimulation) to normal.
Figure.
Cerebrovascular effects of intermittent hypoxia. In response chronic intermittent hypoxia, increased production of endothelin-1 (ET-1) by endothelin-converting enzyme (ECE) activates ETA receptors (ETA-R) resulting in oxidative stress. ET-1 or oxidative stress may also activate components of the inflammatory response. Key consequences of intermittent hypoxia for the vessel wall and mechanisms that regulate cerebral blood flow are shown on the bottom. Nox2 = Nox2 component of NADPH oxidase; O2− = superoxide anion; PiO2 = inspired PO2; NVC = neurovascular coupling. See text for additional details.
Combined with previous reports, the present work suggests that intermittent hypoxia produces endothelial dysfunction in both large and small blood vessels in brain (Figure). While many studies have examined effects of obstructive sleep apnea or intermittent hypoxia on endothelial function in vessels outside the brain previously, the current work appears to be the first demonstration that neurovascular coupling is also affected by intermittent hypoxia. Capone et al9 also provide the first insight into mechanisms by which intermittent hypoxia impairs vasodilator mechanisms in brain, implicating key roles for ET-1, NADPH oxidase, and reactive oxygen species (Figure).
While the present study provides significant insight into mechanisms, unanswered questions remain and new questions emerge. Beyond ET-1, Nox2, and reactive oxygen species, what cell types and specific molecular targets are involved in mediating these effects? Some may be more obvious. For example, microvascular responses to acetylcholine in the brain are mediated by NO produced by endothelial cells. Thus, Nox2-derived superoxide may affect these responses by reducing NO-mediated signaling initiated by endothelial cells. Increases in cerebral blood flow during neurovascular coupling are more complex and likely have NO-dependent and NO-independent components. In addition to neurons, functional hyperemia may also involve astrocytes and/or pericytes in the somatosensory cortex. Beyond endothelium, are all these cells and their relevant cell-specific signaling mechanisms affected by intermittent hypoxia? Vascular structure can be affected by hypoxia, reactive oxygen species, loss of NO bioavailability, as well as ET-1.3,10,11 Does intermittent hypoxia produce inward vascular remodeling (as seen in some models of hypertension) or vascular hypertrophy that encroaches on the vascular lumen? Under some conditions, these types of structural changes can impact vasodilator responses and minimal vascular resistance. In addition to its impact on regulation of vascular tone, are other functions of endothelial cells affected? For example, is blood-brain barrier integrity or transport function impaired in this model? Young male mice were studied, raising the question of whether similar mechanisms are present in female mice or in models of obesity and aging? Obstructive sleep apnea is often associated with obesity and occurs more often in older individuals. Do these same molecules and pathways underlie cerebrovascular abnormalities in models that recapitulate additional features of obstructive sleep apnea?13 Lastly, are effects of intermittent hypoxia on the cerebrovasculature reversible?
What are the implications of these studies? With essentially no energy reserves, the brain needs a constant but adequate baseline perfusion and the ability to rapidly adjust blood flow above and below this baseline in response to changes in cellular activity. Adequate resting blood flow and effective neurovascular coupling mechanisms ensure precise delivery of oxygen, glucose, and other nutrients, as well as efficient removal of metabolic by-products. While is in not clear from the present data whether baseline cerebral blood flow was reduced following intermittent hypoxia, other studies have shown that endothelial dysfunction can decrease resting perfusion due to loss of the influence of basally produced NO (or impairment of other endothelium-dependent mechanisms). Obstructive sleep apnea is a risk factor for cognitive impairment and stroke. A major unanswered question is whether the vascular changes described in the current studies are sufficient to affect cellular (neuronal and glial) function? Do the changes in this model predispose to more severe ischemia, greater impairment of collateral-dependent blood flow, or impair recovery from stroke or other forms or brain injury? While the presence of vascular dysfunction in cerebral blood vessels is analogous to that describe before for the periphery,3,6 the implications can be much greater in brain.
Acknowledgments
SOURCES OF FUNDING
Work by the author is supported by National Institutes of Health grants HL-38901, NS- 24621, HL-62984 and HL-113863.
Footnotes
DISCLOSURES
None
References
- 1.Cipolla MJ. The Cerebral Circulation. In: Granger DN, Granger J, editors. Integrated Systems Physiology: From Molecule to Function. Morgan & Claypool Life Sciences; San Rafael: 2010. pp. 1–59. [Google Scholar]
- 2.Hurn P, Traystman RJ. Changes in Arterial Gas Tension. In: Edvinsson L, Krause D, editors. Cerebral Blood Flow and Metabolism. 2. Chapter 24. Lippincott: Williams & Wilkens; pp. 384–394.pp. 2001 [Google Scholar]
- 3.Levy P, Pepin J-L, Arnaud C, Tamisier R, Borel J-C, Dematteis M, Godin-Ribuot D, Ribuot C. Intermittent hypoxia and sleep-disordered breathing: Current concepts and perspectives. Eur Respir J. 2008;32:1082–1095. doi: 10.1183/09031936.00013308. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veasey S. Insight from animal models into the cognitive consequences of adult sleep-disordered breathing. ILAR J. 2009;50:307–311. doi: 10.1093/ilar.50.3.307. [DOI] [PubMed] [Google Scholar]
- 6.Lurie A. Endothelial dysfunction in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:139–170. doi: 10.1159/000325108. [DOI] [PubMed] [Google Scholar]
- 7.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol. 2004;286:H388–H393. doi: 10.1152/ajpheart.00683.2003. [DOI] [PubMed] [Google Scholar]
- 8.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca2+-activated potassium channels. Circulation Res. 2011;108:1439–1447. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capone C, Faraco G, Coleman C, Young CN, Pickel VM, Anrather J, Davisson R, Iadecola C. Endothelin-1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.112.193672. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faraci FM. Protecting against vascular disease in brain. The Robert M. Berne distinguished lecture. Am J Physiol. 2011;300:H1566–H1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rautureau, Schiffrin EL. Endothelin in hypertension: an update. Curr Opin Nephrol Hypertens. 2012;21:128–136. doi: 10.1097/MNH.0b013e32834f0092. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez Bosc LV, Resta T, Walker B, Kanagy NL. Mechanisms of intermittent hypoxia induced hypertension. J Cell Mol Med. 2010;14:3–17. doi: 10.1111/j.1582-4934.2009.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crossland RF, Durgan DJ, Lloyd EE, Phillips SC, Marrelli SP, Bryan RM. Cerebrovascular consequences of obstructive sleep apnea (Abstract) FASEB J. 2012;26:899.5. [Google Scholar]