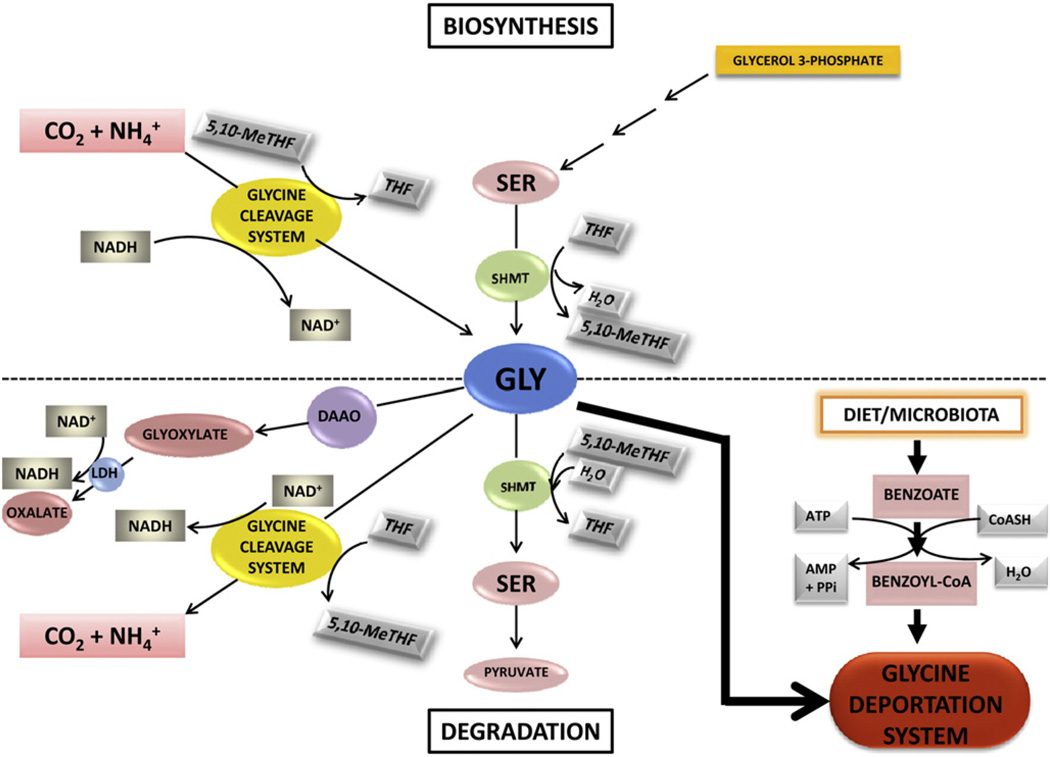
Fig. 1.
The biosynthesis and degradation of glycine. Glycine (GLY) is synthesized from serine (SER) by serine hydroxymethyltransferase (SHMT) using tetrahydrofolate (THF) as cofactor and generating 5,10-methylene-tetrahydrofolate (5,10-MeTHF) and water. GLY is also synthesized de novo from CO2 and by the glycine cleavage system that uses 5,10-MeTHF and NADH and generates THF and NAD+. GLY is metabolically removed by three pathways, specifically the SHMT-mediated synthesis of SER by SHMT, the reverse of the synthetic pathway; the conversion to CO2 and by the glycine cleavage system, utilizing NAD+ and generating NADH, the reverse of the synthetic pathway; and a minor pathway to glyoxylate catalyzed by D-amino acid oxidase (DAAO), and the resulting glyoxylate converted to oxalate by lactate dehydrogenase (LDH), using NAD+ and generating NADH. GLY is also removed by the glycine deportation system by acylation with benzoyl-CoA, yielding N-benzoylglycine (hippuric acid; HA), which is irreversibly excreted into urine. GLY deportation is the only truly irreversible reaction of GLY homeostasis.