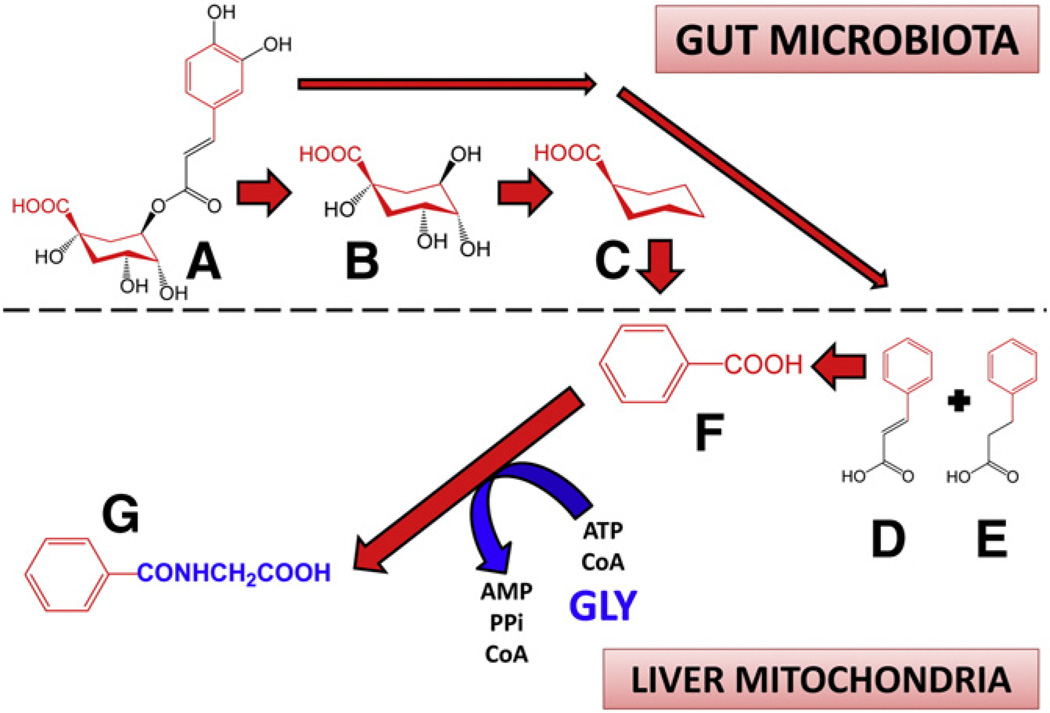
Fig. 4.
Dietary precursors of benzoic acid for the glycine deportation system. Ingested chlorogenic acid (3-caffeoylquinic acid; A) is hydrolyzed by gut microbiota to quinic acid (B) and caffeic acid (not shown). Quinic acid is metabolized to cyclohexane carboxylic acid (C) and this is aromatized by gut microorganisms to benzoic acid (F) which is absorbed into the liver by the hepatic portal vein. Caffeic acid can be metabolized by the microbiota to cinnamic acid (D) and phenylpropionic acid (E), which are also absorbed and taken up by the liver, whereby these are metabolized by β-oxidation to benzoic acid. The benzoic acid formed by these processes is utilized exclusively as the escort molecule for glycine in the glycine deportation system by conversion in hepatic mitochondria to hippuric acid (G). Chemical components of dietary precursors that become benzoic acid are shown in red. Glycine (GLY), both in its free and deported form, is shown in blue.