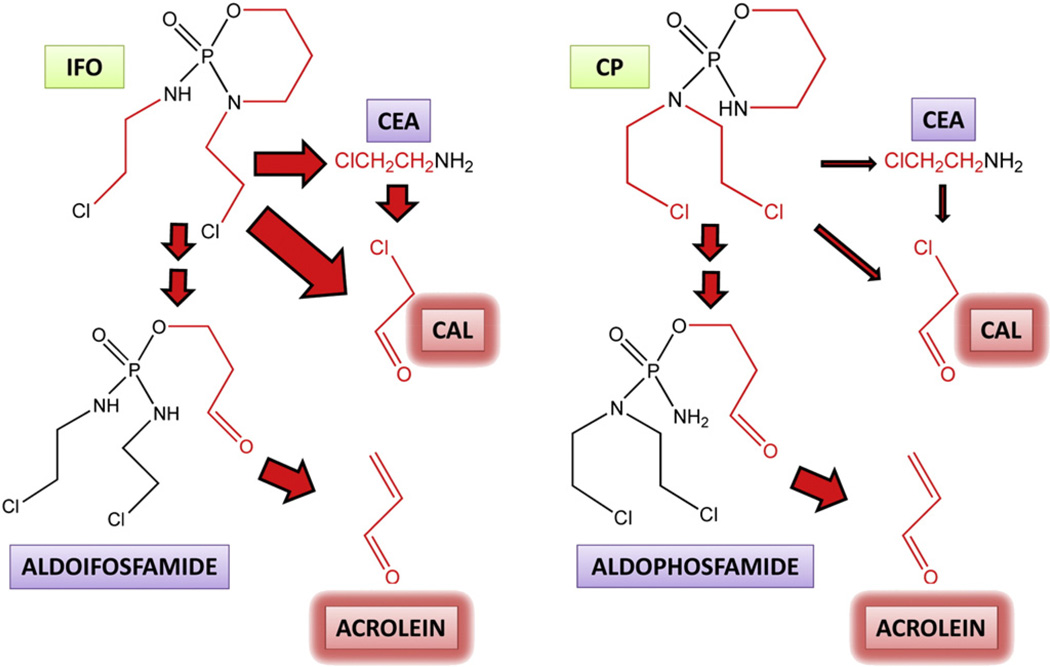
Fig. 5.
The metabolism of ifosfamide and cyclophosphamide to unwanted toxic intermediates. Ifosfamide (IFO) and its structural isomer cyclophosphamide (CP) are both metabolized by cytochrome P450 isozymes to their 4-hydroxy derivatives (not shown), which are ring-opened to the corresponding aldehydes, aldoifosfamide and aldophosphamide, respectively. These aldehyde metabolites both undergo a β-elimination of the highly toxic metabolite acrolein. Cytochrome P450-mediated metabolism of the nitrogen mustard side-chains also occurs leading to the toxic metabolite chloroacetaldehyde (CAL), either directly, or via 2-chloroethylamine (CEA), with the participation of amine oxidases. Acrolein is believed to cause urotoxicity and CAL has been proposed to cause neurotoxicity. Arrow size indicates the relative flux down different pathways and therefore that CAL formation is considerably greater with IFO than with CP. Chemical components of IFO and CP that become the toxic aldehydes acrolein and CAL are shown in red.