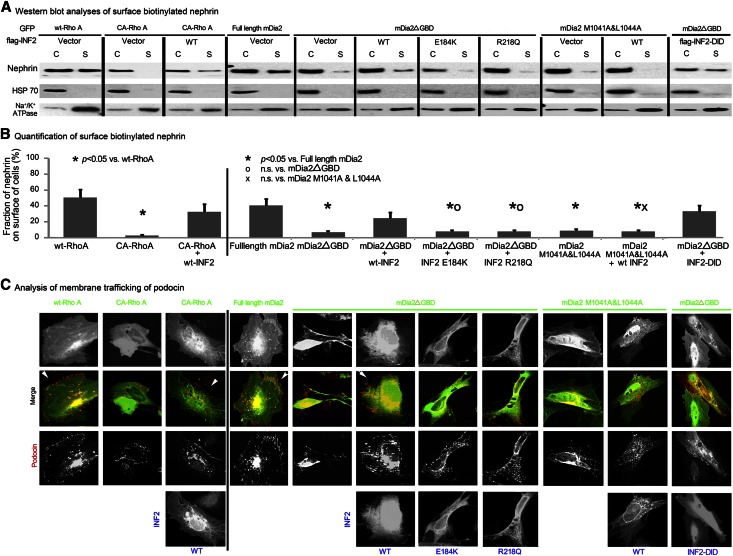
Figure 6.
Mutations disassociating INF2 from mDia impair INF2’s ability to rescue lamellipodial trafficking in response to Rho/mDia activation. Podocytes were transfected with plasmids encoding different forms of Rho (wt or CA-RhoA, GFP fusion), with or without cotransfection of WT-INF2 or were cotransfected with plasmids encoding different forms of INF2 (wt full length, E184K full length, or R218Q full-length, WT-INF2-DID) and different forms of mDia2 (full-length mDia2 as an autoinhibited form, mDia2ΔGBD and mDia2 1041A&1044A mutant as two forms of disinhibited or constitutively active mDia2). (A) The cytosol (C) and the surface biotinylated nephrin (S) in cells with different treatments was measured by Western blotting. (B) Surface biotinylation was used to measure the surface membrane trafficking of nephrin. HSP 70 and Na+/K+ ATPase were used as controls for unbiotinylated and surface-biotinylated proteins, respectively. The membrane trafficking of nephrin was quantified as the fraction of biotinylated nephrin (S/S+C)% in three independent experiments of each group; data were expressed as mean ± standard deviation. The difference among groups was analyzed by using one-way ANOVA and least significant difference test. (C) Podocin trafficking in cells with different treatments was illustrated by immunostain.