Table 1.
Human serine hydrolase inhibitors approved for clinical use.
| Target | Compound | Structure | Company | Ref(s) | Indication |
|---|---|---|---|---|---|
| ACHE | Rivastigmine (Exelon) |
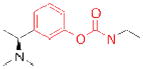
|
Novartis | 29 | Alzheimer’s - associated dementia |
| Donepezil (Aricept) |
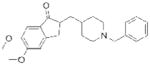
|
Eisai | 69 | ||
| Galantamine (Razadyne) |
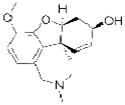
|
Ortho-McNeil Janssen | 72 | ||
| Tacrine (Cognex) |
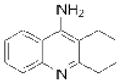
|
Shionogi | 66 | ||
| DPP4 | Sitagliptin (Januvia) |
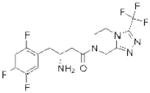
|
Merck | 93 | Type II diabetes |
| Saxagliptin (Onglyza) |
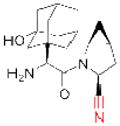
|
Bristol Myers Squibb | 92 | ||
| Linagliptin (Tradjenta) |
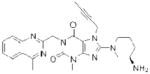
|
Boehringer Ingelheim | 94 | ||
| Vildagliptin* (Zomelis) |
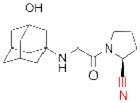
|
Novartis | 31 | ||
| Alogliptin* (Nesina) |
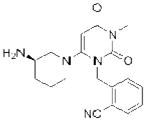
|
Takeda | 86 | ||
| Pancreatic/ gastric lipases | Orlistat (Xenical;Alli) |
|
Roche; GlaxoSmithKline | 19, 32 | Obesity |
| Thrombin | Dabigatran etexilate (Pradaxa) |
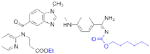
|
Boehringer Ingelheim | 46, 47 | Thrombosis |
| Argatroban (Novastan) |
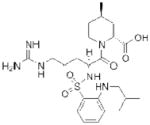
|
GlaxoSmithKline Mitsubishi Pharma | 42 | ||
| Factor Xa | Rivaroxaban (Xarelto) |
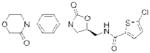
|
Bayer | 54, 59 | Thrombosis |
| Human neutrophil elastase | Sivelestat* (Elaspol) |
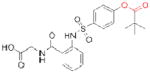
|
Ono | 33 | Respiratory disease |
The electrophilic moieties of each compound, if applicable, are colored red. The prodrug portions of dabigatran etexilate are colored blue.
Not yet approved in the United States.
