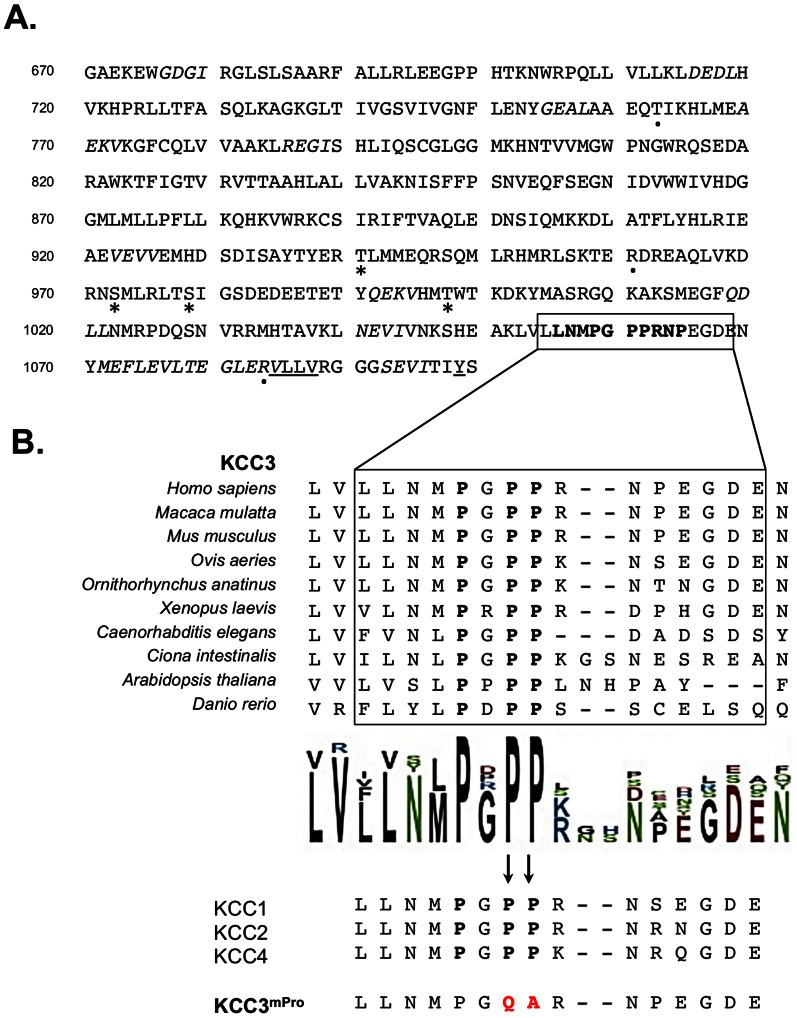
Figure 1. Identification of a functional proline-rich motif in the C-terminal domain of KCC3.
(A) Protein sequence of the KCC3 C-terminal domain. The C-terminus of KCC3 contains a distal poly-proline motif indicated in bold characters which is predicted to be a binding site for SH3 domains with a non-canonical class I recognition specificity (ELM motif data base). The C-terminus also contains a hydrophobic tetrad and a predicted Tyrosine residue (both underlined) involved in cation-chloride co-transporter trafficking. Predicted PDZ domains interacting motifs are shown in italic. Three HMSN/ACC non-sense mutations are indicated by a dot (.). The phosphorylated sites are indicated by a star (*). (B) KCC3 C-terminal domain is highly conserved in the proline-rich region. The motif is conserved at 100% between KCC3 homologs but also between KCC3 orthologs KCC1, KCC2 and KCC4. The proline stretch was mutated in order to generate mPro mutant forms of KCC3 polypeptide and protein.