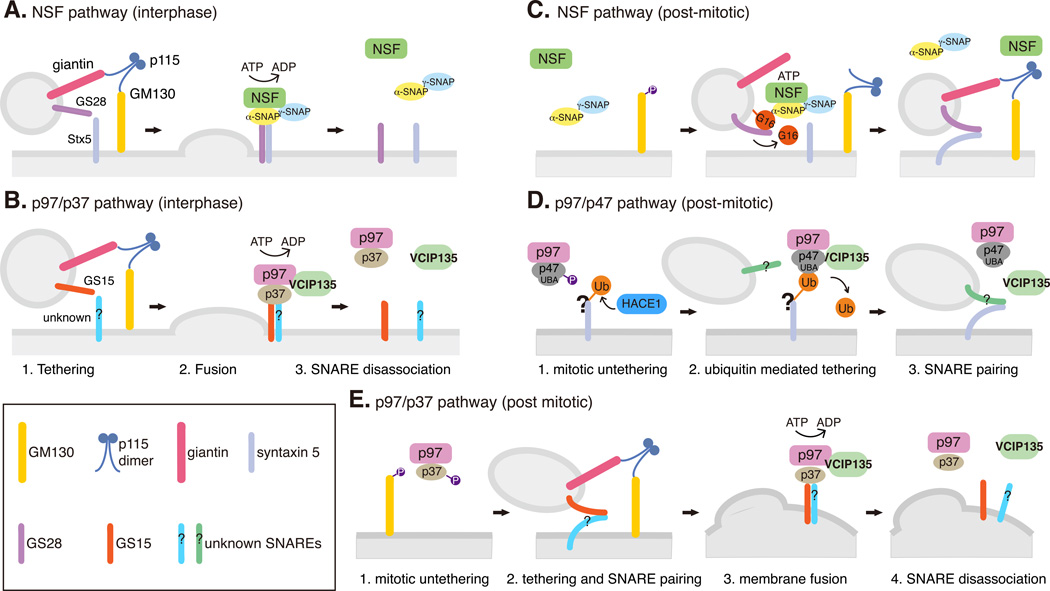
Fig. 2. NSF and p97 pathways in interphase Golgi membrane fusion and post-mitotic Golgi reassembly.
A-B. Interphase Golgi membrane fusion mediated by NSF (A) and p97/p37 (B). Vesicles or Golgi membranes are tethered by the p115/GM130 complex, which facilitates SNARE-mediated fusion. The SNARE complexes are disassociated by NSF or p97 by ATP hydrolysis to allow next round vesicle fusion.
C-D. Ubiquitin-mediated post-mitotic Golgi fusion by NSF (C) and p97/p47 (D). During mitosis, GM130 phosphorylation interrupts its interaction with p115 and facilitates Golgi vesiculation. After mitosis, ubiquitination operates as a general mechanism for Golgi cisternae regrowth. In the NSF pathway (C), GATE-16, a ubiquitin-like protein, recruits NSF onto the membranes. NSF then catalyzes GATE-16 to form a complex with GS28 and inhibits GS28-syntaxin 5 interaction in an ATP-hydrolysis independent manner. GATE-16 is released from the membrane by an unknown mechanism to allow SNARE complex assembly and membrane fusion. In the p97/p47 pathway (D), HACE1 attaches ubiquitin onto unknown substrates on the Golgi membranes during mitosis; ubiquitination of these Golgi proteins may inhibit vesicle fusion. In late mitosis, the ubiquitin bound to a Golgi protein interacts with the UBA domain of p47 and recruits the p97/p47/VCIP135 complex onto Golgi vesicles. VCIP135 removes ubiquitin from Golgi proteins and allows p97/p47 to fuse the Golgi fragments into new cisternae. How p97/p47 regulates SNARE pairing and membrane fusion in this process is still unclear.
E. Ubiquitin is not involved in post-mitotic Golgi fusion by p97/p37. During mitosis, p37 is phosphorylated and thus membrane fusion is inhibited. After mitosis, dephosphorylation of p37 allows membrane fusion similar to that in interphase (B). In this pathway, SNARE complex is disassembled by p97 ATP hydrolysis, while the p97/p37 complex is disassembled by VCIP135.