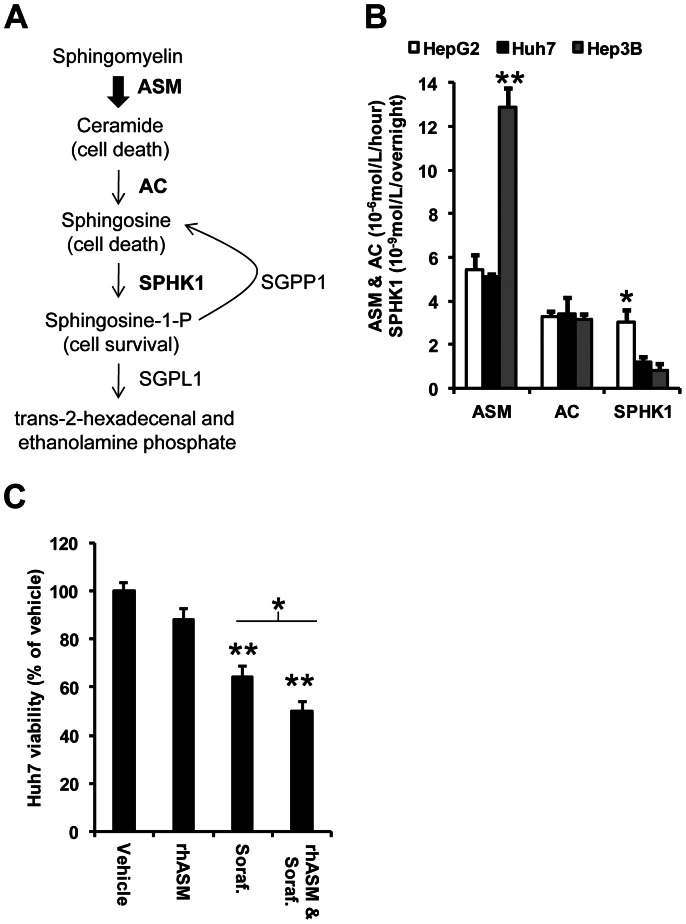
Figure 1. Rationale and selection of human hepatoma cells.
(A) ASM drives the production of pro-apoptotic ceramide through the hydrolysis of sphingomyelin, which is converted to sphingosine by ceramidases such as acid ceramidase (AC). Sphingosine kinase 1 (SPHK1) then phosphorylates sphingosine to sphingosine-1-phosphate (S1P), which is converted back to sphingosine by S1P-phosphatase (SGPP1) or metabolized by S1P lyase 1 (SGPL1). (B) Activity of ASM in Hep3B cells was significantly higher (ANOVA, df (2,6), F = 48.49, p<0.001) than in Huh7 (Tukey's post hoc test p<0.001, **) and HepG2 cells (Tukey's post hoc test p<0.001). AC was similar across all cell lines, but HepG2 cells had significantly higher SPHK1 activity (ANOVA, df (2,6), F = 8.68, p = 0.017, *) than Huh7 (Tukey's post hoc test, p = 0.041) and Hep3B (Tukey's post hoc test, p = 0.019). (C) Huh7 cells were selected for further studies and their viability tested at pH 6.5 (see Methods) in the presence of 500 µg/mL rhASM, 3 µM sorafenib, or the combination of rhASM and sorafenib at 48 hours. Sorafenib (Dunnett's post hoc test p<0.001, **) and combined rhASM/sorafenib (Dunnett's post hoc test p<0.001, **) treated cells had significantly lower viability than control cells (ANOVA, df (3,38), F = 26.47, p<0.001). rhASM was not significantly different from control (p = 0.118). The rhASM and sorafenib combination exhibited significantly lower viability compared to sorafenib alone (t-test, 1-sided, *p<0.05, **p<0.001).