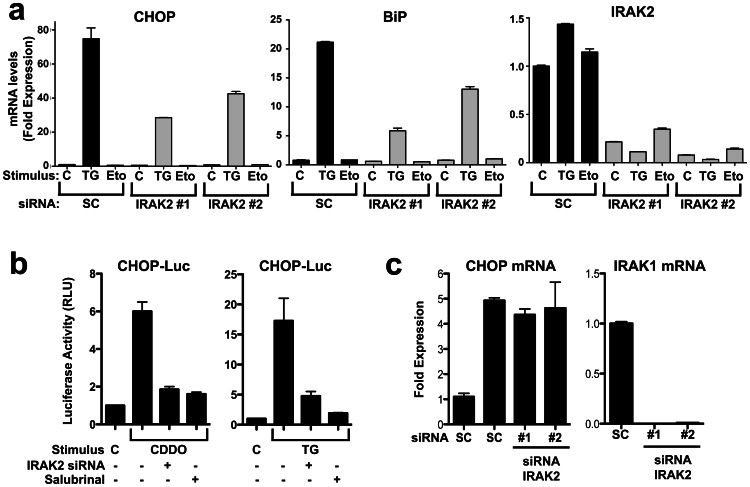
Figure 2. IRAK2 knock down inhibits UPR signaling.
(a) PPC1 cells were transiently transfected with 2 independent IRAK2 siRNAs (gray bars) or scrambled controls (SC) (black bars). After 2 days, cells were cultured with 5 µM Thapsigargin (TG) or 100 µM Etoposide (Eto) for 3 hrs then total RNA was extracted. Relative levels of mRNAs encoding CHOP (left), BiP/Grp78 (middle), and IRAK2 (right) were compared by qRT-PCR and displayed as ratios relative to a housekeeping gene (cyclophilin). Data are mean±SD, n = 3. (b) PPC1 cells were transfected with IRAK2 siRNA (+) or scrambled (Sc) siRNA (−). After 2 days, reporter gene plasmids CHOP-Luc and Renilla-Luc were transfected. Then 4–6 hrs later, cells were cultured without (C) or with 5 µM CDDO-Im (CDDO) or TG, with or without the ER stress inhibitor Salubrinal for 6 hrs. Luciferase activity was subsequently measured, normalizing firefly Luc driven from the CHOP gene promoter relative to Renilla Luc and expressing data as fold-induction relative to untreated cells transfected with SC control siRNA (mean±SD, n = 3). (c) Cells were transfected with either scrambled or IRAK1 siRNAs and subsequently cultured without (−) or with (+) 5 µM TG for 3 hrs. Then, RNA was extracted and CHOP and IRAK1 mRNA expression were measured by qRT-PCR (normalized to Cyclophilin). Data are expressed as fold-induction relative to untreated SC control transfected cells (mean±SD, n = 3).