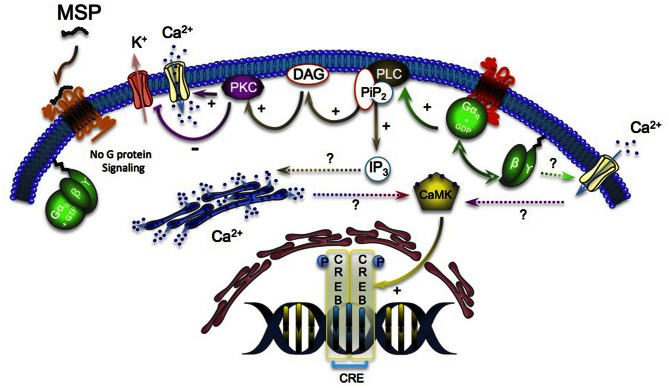
Figure 1.
GHS-R1a constitutive activity leads to the dissociation of α and βγ subunits of heterotrimeric G-proteins. The free Gq subunit activates the PLC that cleaves PiP2 into IP3 and DAG. DAG activates the PKC, which in turn actives the Ca2+ channels and inhibits the K+ channels (continuous line). The effects of IP3 on the intracellular Ca2+ mobilization and the origin of the Ca2+ that activates the Ca2+ calmodulin kinase (CaMK) remain unclear and will need further investigations (dotted line). The binding of the inverse agonist MSP to the GHS-R1a inhibits the G-protein signaling and decreases the IP3 (via PLC) and CRE pathway (via phosphorylated CREB) constitutive activation.