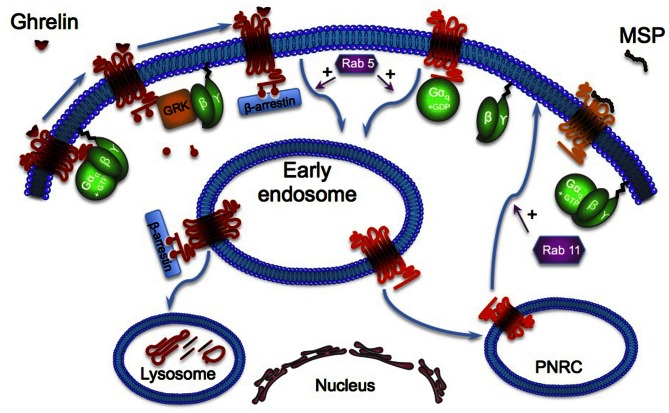
Figure 2.
GHS-R1a activation by its endogenous ligand ghrelin leads to the dissociation of α and βγ subunits of heterotrimeric G-proteins. The βγ dimer recruits G-protein-receptor kinases (such as GRK2 or GRK3) to the receptor, where they phosphorylate the agonist-bound receptor. These phosphorylations lead to the recruitment of β-arrestins and activation of the monomeric G-protein RAb5 that targets the receptor to early endosomes. Then the receptor is addressed to the lysosome for degradation. GHS-R1a constitutive activation recruits Rab5 without activation of the GRK, inducing subsequent phosphorylation and β-arrestin recruitment. This constitutive activation of Rab5 explains the receptor ligand-independent internalization that is first addressed to the early endosome then to the PNRC (perinuclear recycling compartment) where it activates the monomeric G-protein Rab11.