Abstract
Background. Millions of individuals being treated for human immunodeficiency virus (HIV) live in malaria-endemic areas, but the effects of these treatments on malaria transmission are unknown. While drugs like HIV protease inhibitors (PIs) and trimethoprim-sulfamethoxazole (TMP-SMX) have known activity against parasites during liver or asexual blood stages, their effects on transmission stages require further study.
Methods. The HIV PIs lopinavir and saquinavir, the nonnucleoside reverse-transcriptase inhibitor nevirapine, and the antibiotic TMP-SMX were assessed for activity against Plasmodium falciparum transmission stages. The alamarBlue assay was used to determine the effects of drugs on gametocyte viability, and exflagellation was assessed to determine the effects of drugs on gametocyte maturation. The effects of drug on transmission were assessed by calculating the mosquito oocyst count as a marker for infectivity, using standard membrane feeding assays.
Results. Lopinavir and saquinavir have gametocytocidal and transmission blocking activities at or approaching clinically relevant treatment levels, while nevirapine does not. TMP-SMX is not gametocytocidal, but at prophylactic levels it blocks transmission.
Conclusions. Specific HIV treatments have gametocyte killing and transmission-blocking effects. Clinical studies are warranted to evaluate these findings and their potential impact on eradication efforts.
Keywords: HIV, malaria, antiretrovirals, TMP-SMX, gametocytes, transmission
When human immunodeficiency virus (HIV) and malaria parasites are both present in an individual, they exert copathogenic effects [1]. This interaction may contribute to increased malaria transmission. The World Health Organization recommends HIV management with combination antiretroviral therapy, composed of a nonnucleoside reverse-transcriptase inhibitor (NNRTI) and a nucleoside reverse-transcriptase inhibitor (NRTI) as first-line therapy, with second-line therapy including an HIV protease inhibitor (PI) and 2 NRTIs [2]. HIV PIs affect Plasmodium development in vitro and in animal models [4, 5]. Clinical studies are beginning to examine HIV PI impact on malaria in children [6]. Separately, the antibiotic trimethoprim-sulfamethoxazole (TMP-SMX) is frequently used in HIV-exposed infants and HIV-infected patients [3]. TMP-SMX also reduces the incidence of clinical malaria in areas of varying transmission intensities and where the mutation conferring antifolate resistance among malaria parasites is prevalent [1, 7]. Given the use of these drugs in HIV management in malaria-endemic areas, the effect of different antiretrovirals and TMP-SMX prophylaxis on malaria transmission requires further study.
The malaria parasite has several distinct life cycle stages: preerythrocytic stage (sporozoite and liver), asexual and sexual erythrocytic stages, and mosquito stages. Sexual stage parasites, or gametocytes, are transmitted to the mosquito, where they develop into oocysts and then sporozoites, the infective form of the parasite. Gametocytes are usually found in the blood of an infected patient, even after treatment, as most treatments target the asexual stage, which is associated with symptoms. These gametocytes serve to perpetuate the cycle of transmission [8].
HIV PIs have antimalarial effects on erythrocytic asexual stages [5], and certain HIV PIs have been shown to affect gametocytogenesis and reduce gametocyte viability [9], but their specific effects on early and late stage gametocytes and transmission require further investigation. Additionally, while NNRTIs have little to no effect on liver or asexual stage parasites at clinically relevant concentrations [10–12], their effect on malaria gametocytes or transmission requires further investigation. Last, TMP-SMX has been shown to reduce clinical episodes of malaria in HIV-infected and HIV-exposed patients [1, 13], but the influence of TMP-SMX prophylaxis on transmission requires further study.
The effect of drugs on transmission may involve killing gametocytes or blocking parasite transmission to or development within the mosquito [8]. Using an assay that screens for gametocytocidal compounds [14], we evaluated a panel of HIV antiretrovirals (HIV PIs and an NNRTI) and TMP-SMX for their effect on P. falciparum gametocyte viability. We then evaluated a selection of these compounds for transmission-blocking effects in a standard membrane-feeding assay (MFA) [15].
METHODS
Parasites
P. falciparum strains NF54 and 3D7, a clone of the parental isolate NF54, were used in all assays. Gametocyte stage was determined by morphology, as previously described [16].
HIV Drug Preparation
HIV PIs were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. Stocks were prepared in dimethyl sulfoxide (DMSO), and final concentrations for experiments were prepared by diluting stock with culture medium. For all experiments, DMSO in culture medium alone was added to the control wells. Lopinavir-ritonavir was tested in a 4:1 ratio based on molecular weight to mimic the clinical formulation, and ritonavir was tested alone or in combination with lopinavir at a range of 1–20 µM (the lower concentrations based on the 4:1 clinical formulation). Saquinavir and lopinavir were tested at 5–20 µM. Nevirapine concentrations ranged from 3.75 µM to 15 µM, and TMP-SMX concentrations ranged from 20 µM to 40 µM. Drug concentrations were based on levels achieved by standard drug regimens in HIV-infected patients receiving antiretroviral treatment and TMP-SMX prophylaxis [11, 17–20]. Drugs were tested across a wider range of concentrations if they had significant effects at the higher concentrations on initial screens.
Gametocyte Viability Assessment
Drug-treated and control cultures of 3D7 P. falciparum early stage (stage II–III) gametocytes and late stage (stage III–V) gametocytes were compared using the alamarBlue indicator assay, which reflects metabolic activity and, thus, viability of gametocytes [14]. This assay was performed as described previously, with few modifications [14]. Briefly, parasites were treated with 50 mM N-acetyl glucosamine to kill asexual stage parasites on days 6–8 and 9–11 for early and late stage gametocytes, respectively. Infected red blood cells (RBCs) were enriched by Percoll density-gradient centrifugation on days 9 and 12 for early and late stages, respectively. Cultures were adjusted to 10% gametocytemia, and assay plates were set up on days 10 and 13 for early and late stages, respectively. At this time, gametocytes were mixed with DMSO-diluted HIV drug; with epoxomicin, a proteasome inhibitor that kills all gametocytes (a positive control, which provided a background measurement attributable to RBCs only); or with DMSO alone (negative control). The gametocyte mixtures were then cultivated under standard conditions until days 13 and 16 for early and late stages, respectively. For the early and late stages, alamarBlue was then added on days 13 and 16, and fluorescence was detected on days 14 and 17. Background fluorescence from RBCs was subtracted using epoxomicin values. Z-factor values were calculated to account for well-to-well variability [21]. Three to four experiments were conducted for early and late gametocytes, with each experiment run in duplicate or triplicate.
To record the morphologic appearance of treated and control early and late stage gametocytes, Giemsa-stained smears were prepared after alamarBlue detection, and pictures of smears were taken (original magnification, ×1000).
Transmission Assessment
Drugs may affect transmission at the level of the gametocyte or mosquito infectivity. Therefore, we then assessed transmission blocking effect by means of the mosquito oocyst count, which reflects mosquito infectivity, in mosquitoes that fed on drug-treated or control parasite cultures. Standard membrane feeding assays (MFAs) were carried out as previously described, with few modifications [15]. Briefly, cultures containing NF54 strain day 15–16 stage V gametocytes were diluted with O + RBC and heat-inactivated O+ serum to achieve a 0.15% ± 0.05% stage V gametocyte concentration in 50% hematocrit. Then, 200-µL aliquots mixed with 60 µL of phosphate-buffered saline were mixed with DMSO-diluted HIV drugs or 1 µM primaquine, which corresponds to the gametocytocidal primaquine dose levels achieved for P. falciparum [22–24], or with DMSO alone. After mixing, cultures were immediately fed to 3–5-day-old Anopheles stephensi mosquitoes. Eight days later, mercurochrome-stained oocysts were counted on midguts of ≥20 dissected mosquitoes per condition, and the percentage inhibition of oocyst development was calculated. To assess prolonged treatment and to distinguish drug effects on gametocyte versus mosquito stages, gametocytes were also exposed to drugs for 72 hours prior to performance of MFAs.
Gametocyte Maturation Assessment
Mosquito infectivity requires maturation and development of the parasite. We tested whether the HIV PIs affected gametocyte maturation in the above experiments by assessing exflagellation after 24, 48, and 72 hours of incubation with drug, as previously described with few modifications [25]. Briefly, all culture and reagent materials were kept at 37°C, and 1–1.5 mL of culture was centrifuged. After supernatant was aspirated, an equal volume of O+ serum was added to the pellet and mixed. After 10 minutes at room temperature, 10 µL of pellet-serum mix was placed on a slide, covered with a coverslip, and examined under a light microscope (original magnification, ×400). The average number of exflagellation centers per field was counted in ≥7 fields.
Statistics
For all experimental analysis, data were normalized to the DMSO negative control. One-way analysis of variance was used to compare treated parasites to control, taking into account multiple replicates. The Tukey method was used to identify statistically significant differences at the P < .05 level, and P values are associated with comparisons of mean values of treated samples to the DMSO control. Statistical analyses were performed using GraphPad Prism software (version 5).
RESULTS
HIV PIs, but Not the NNRTI or TMP-SMX, Kill Early and Late Stage Gametocytes
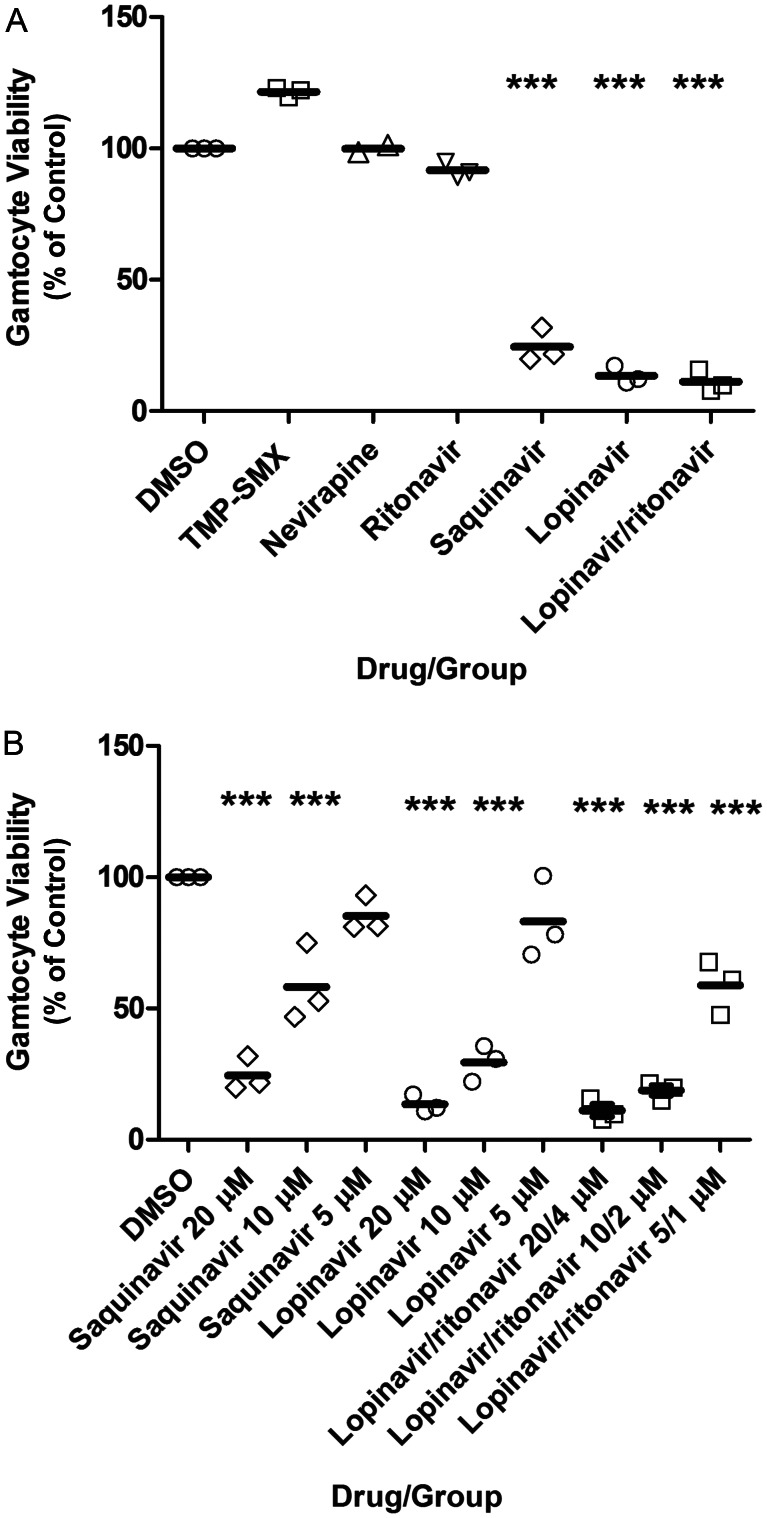
Assessment of drug effect on transmission includes assessment of drug effect on gametocyte viability, which we measured with the alamarBlue assay (Figure 1). Neither TMP-SMX, nevirapine, nor ritonavir alone significantly reduced early stage gametocytes (P > .05). In contrast, saquinavir at 20 µM and 10 µM significantly reduced gametocyte viability (P < .001), but not at 5 µM (P > .05). Lopinavir at 20 µM and 10 µM reduced viability (P < .001), but not at 5 µM (P > .05). Lopinavir/ritonavir at 20 µM/4 µM, 10 µM/2 µM, and 5 µM/1 µM significantly reduced viability (P < .001). Treatments with lopinavir alone compared with lopinavir/ritonavir were not significantly different from each other (P > .05), except at the lowest concentration (P < .05). The mean well-to-well Z′-factor (±SD) was 0.88 ± 0.02.
Figure 1.
Human immunodeficiency virus (HIV) drug effect on early stage gametocyte viability, as assessed by the alamarBlue assay. Day 10 gametocytes were mixed with dimethyl sulfoxide (DMSO)–diluted HIV drug or DMSO alone, and viability was assessed on day 14. Shown is the mean percentage (±SD) of control fluorescence for 3 independent experiments. A, The highest concentrations for each drug tested is shown. B, The full range of drug concentrations tested with significant effect is shown. The mean well-to-well Z′-factor (±SD) was 0.88 ± 0.02. ***P < .001, vs control. Abbreviation: TMP-SMX, trimethoprim-sulfamethoxazole.
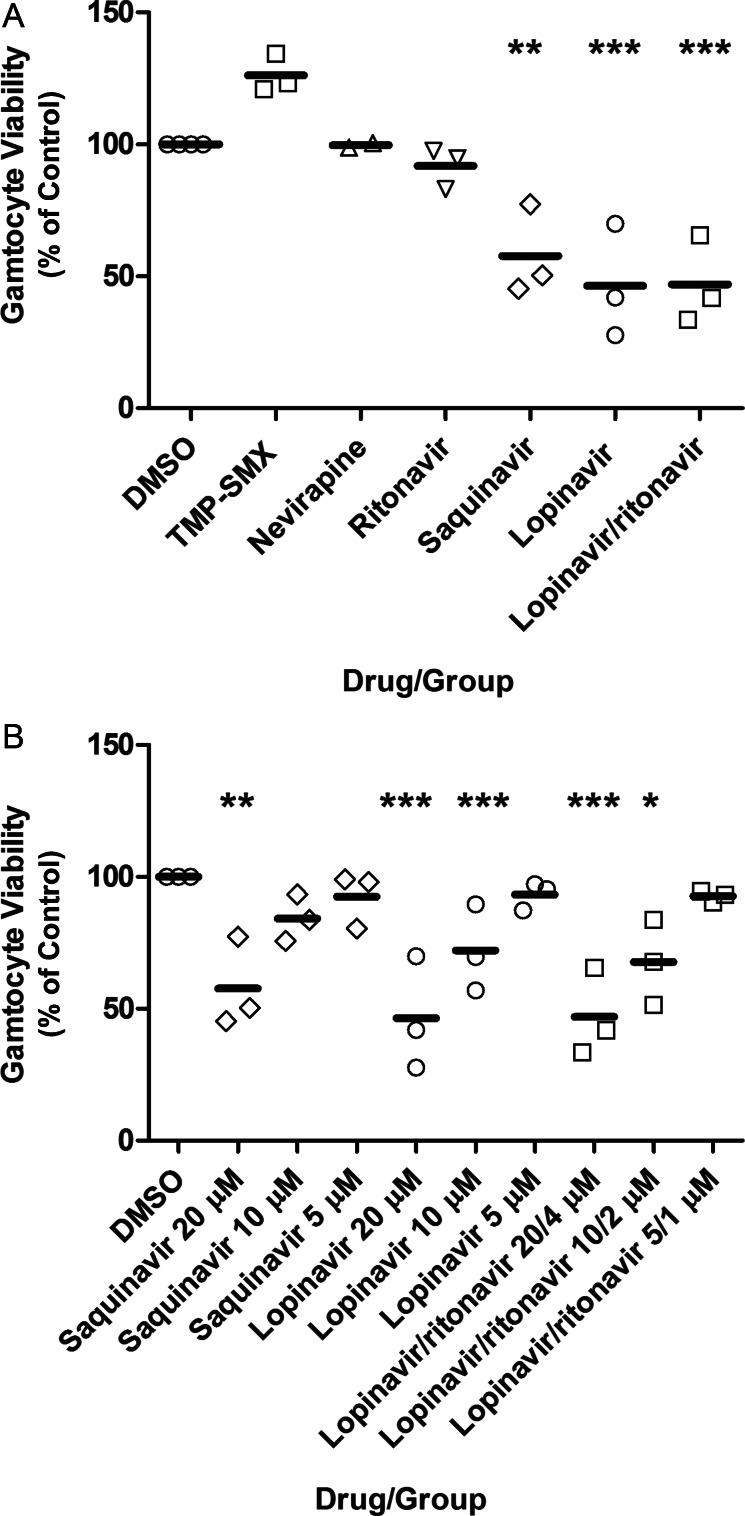
Late stage gametocyte drug treatment results (Figure 2) paralleled the early stage gametocyte treatment results described above. Neither TMP-SMX, nevirapine, nor ritonavir alone significantly reduced late stage gametocyte viability (P > .05). In contrast, saquinavir at 20 µM significantly reduced gametocyte viability (P < .01), but not at 10 µM or 5 µM (P > .05). Lopinavir at 20 µM and 10 µM reduced viability (P < .001), but not at 5 µM (P > .05). Lopinavir/ritonavir at 20 µM/4 µM and 10 µM/2 µM reduced viability (P < .001 and P < .05, respectively), but not at 5 µM/1 µM (P > .05). Treatments with lopinavir alone compared with lopinavir/ritonavir did not differ at any concentration (P > .05). The mean well-to-well Z′-factor (±SD) was 0.79 ± 0.11.
Figure 2.
Human immunodeficiency virus (HIV) drug effect on late stage gametocyte viability, as assessed by the alamarBlue assay. Day 13 gametocytes were mixed with dimethyl sulfoxide (DMSO)–diluted HIV drug or DMSO alone, with viability assessed on day 17. Shown is the mean percentage (±standard error of the mean) of control fluorescence for 4 independent experiments. A, The highest concentrations for each drug tested is shown. B, The full range of drugs concentrations for the HIV protease inhibitors lopinavir and ritonavir is shown. The mean well-to-well Z′-factor (±SD) was 0.79 ± 0.11. **P < .01 and ***P < .001, vs control. Abbreviation: TMP-SMX, trimethoprim-sulfamethoxazole.
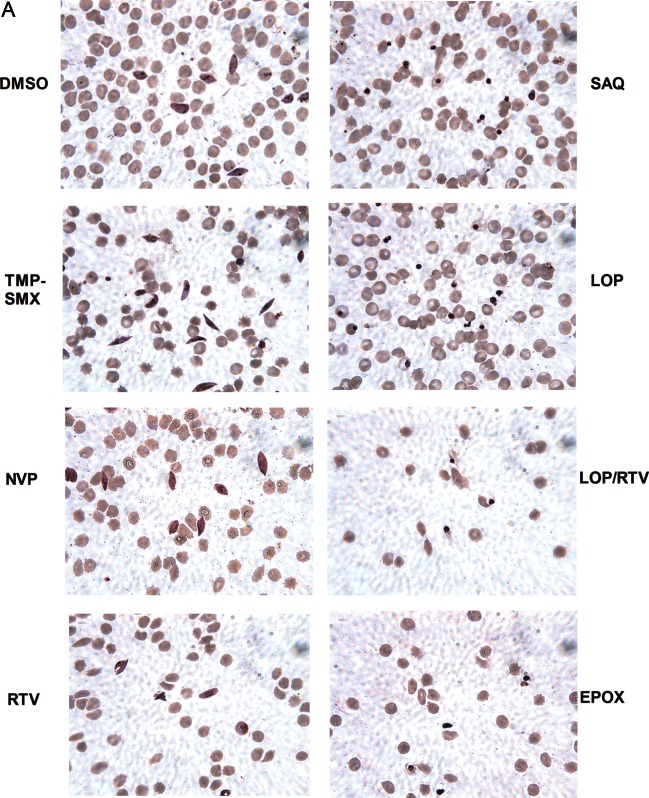
To evaluate the morphology of the treated gametocytes, Giemsa-stained smears were prepared. Saquinavir, lopinavir, ritonavir, and epoxomicin treatment altered early (Figure 3A) and late stage (Figure 3B) gametocyte morphology (pyknotic cells, decreased nucleus size relative to cytoplasm), whereas nevirapine and TMP-SMX did not.
Figure 3.
Human immunodeficiency virus (HIV) drug effect on gametocytes, by optical microscopy. Images of representative Giemsa-stained smears of early stage (A) and late stage (B) gametocytes are shown, drawn from assays described in Figure 1A and 2A, respectively. Treatment with dimethyl sulfoxide (DMSO) alone, trimethoprim-sulfamethoxazole (TMP-SMX), or nevirapine (NVP) did not affect gametocyte morphology. In contrast, treatment with ritonavir (RTV), saquinavir (SAQ), lopinavir (LPV), lopinavir/ritonavir (LOP/RTV), and a known gametocytocidal agent, epoxomicin (EPOX), resulted in altered gametocyte morphology (pyknotic cells and decreased nucleus size relative to cytoplasm).
HIV PIs, but Not the NNRTI or TMP-SMX, Reduce Oocyst Count
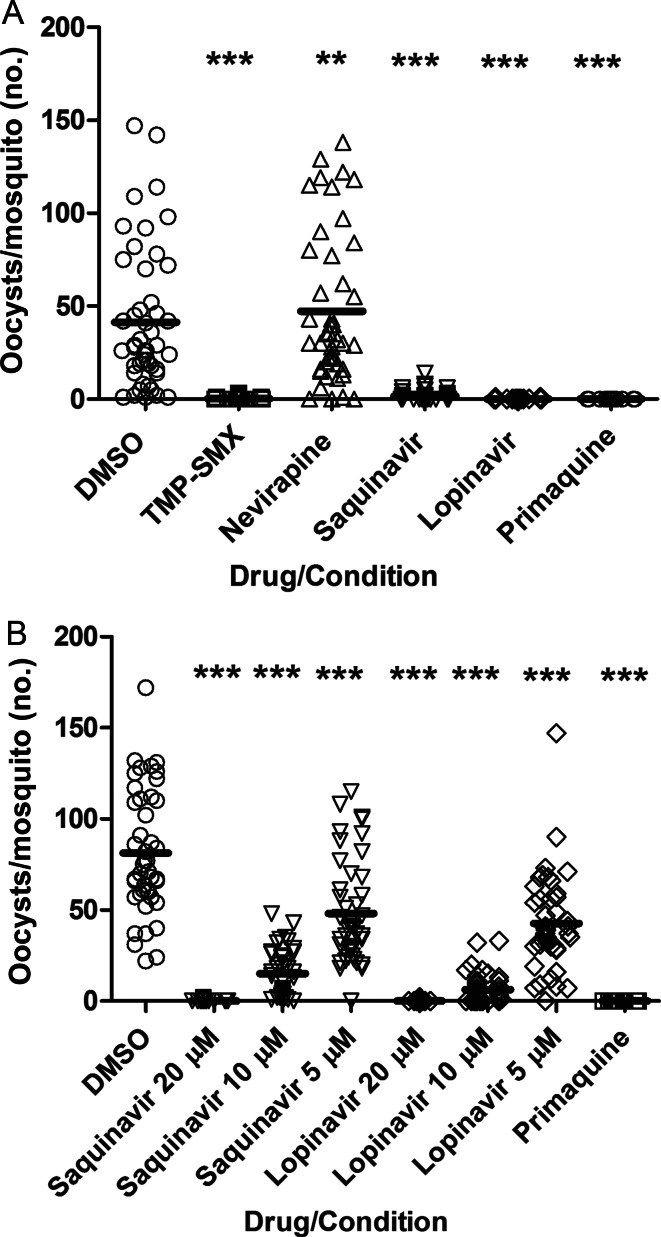
To directly assess drug effect on transmission to the mosquito, stage V gametocyte–containing cultures were mixed with DMSO-diluted HIV drugs, P. falciparum–gametocytocidal doses of primaquine [22–24], or DMSO alone and then fed to A. stephensi mosquitoes. The percentage inhibition of oocyst development in these mosquitoes was measured. For MFA, all drugs were tested at a range of concentrations, including clinically relevant concentrations.
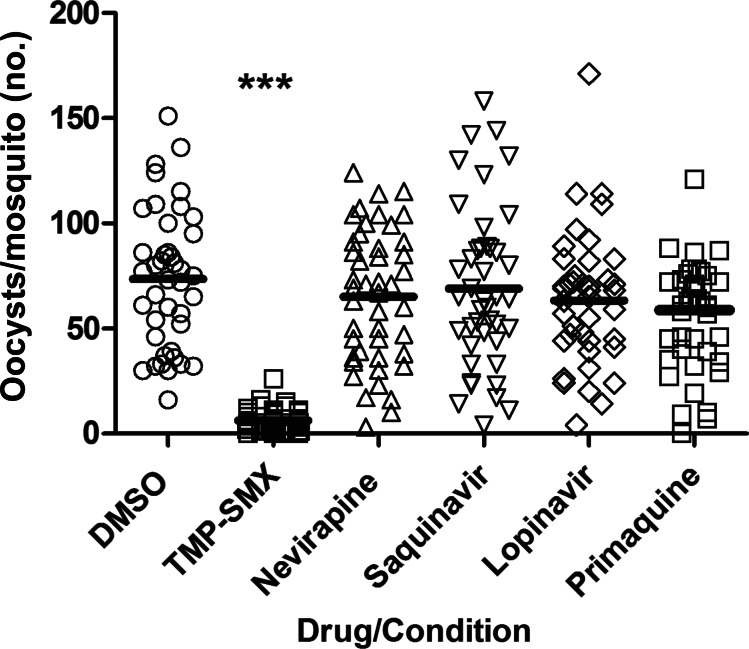
When MFA was undertaken immediately after parasite-drug exposure, only TMP-SMX 40 µM reduced the oocyst count (P < .001; Figure 4). However, in MFA performed after prolonged parasite-drug exposure, TMP-SMX at 40 µM, lopinavir at 20 µM, and saquinavir at 20 µM each reduced the oocyst count (P < .001), while nevirapine at 15 µM increased the oocyst count (P < .01), and primaquine had no effect (P > .05; Figure 5A). Saquinavir and lopinavir were then tested at lower concentrations that included clinically relevant levels (20 µM, 10 µM, and 5 µM), and both drugs significantly reduced oocyst count after prolonged exposure (P < .001 for both comparisons; Figure 5B).
Figure 4.
Human immunodeficiency virus (HIV) drug effect on transmission immediately after drug exposure. Day 15 cultures containing stage V gametocytes were mixed with dimethyl sulfoxide (DMSO)–diluted HIV drugs, primaquine, or DMSO alone and immediately fed to 3–5-day-old Anopheles stephensi mosquitoes. Mercurochrome-stained oocysts were counted on the midguts of ≥20 dissected mosquitoes. Pooled results of 2 independent experiments are shown. ***P < .001, vs control. Abbreviation: TMP-SMX, trimethoprim-sulfamethoxazole.
Figure 5.
Human immunodeficiency virus (HIV) drug effect on transmission after prolonged parasite-drug exposure. From day 13 of culture, stage V gametocytes were incubated in dimethyl sulfoxide (DMSO)–diluted HIV drugs, primaquine, or DMSO alone, each added daily, and were then fed to 3–5-day-old Anopheles stephensi mosquitoes on day 16. Mercurochrome-stained oocysts were counted on the midguts of ≥20 dissected mosquitoes per condition 8 days later. Pooled results of 2 independent experiments are shown. A, The highest concentrations for each drug tested are shown. B, The full range of tested concentrations of the HIV protease inhibitor lopinavir and ritonavir are shown. **P < .01 and ***P < .001, vs control. Abbreviation: TMP-SMX, trimethoprim-sulfamethoxazole.
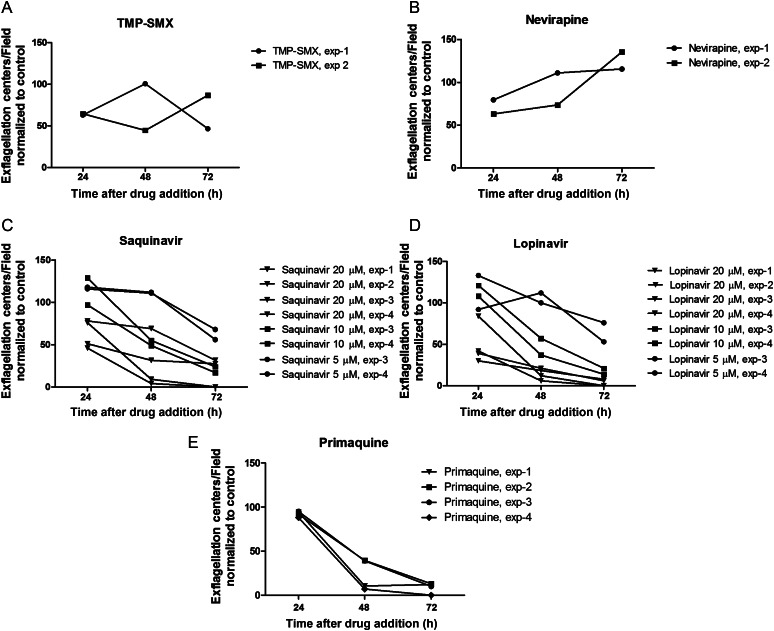
HIV PIs, but Not the NNRTI or TMP-SMX, Inhibit Gametocyte Exflagellation/Maturation
Gametocytes must mature to be taken up by the mosquito in an infectious blood meal, and the measure of this maturity in male gametocytes is exflagellation, which takes place prior to fertilization, ookinete formation, and oocyst formation [26]. To pinpoint the time at which HIV PIs might exert their effect within transmission, we evaluated the effect of HIV PIs on exflagellation. In the MFA in which prolonged parasite-drug exposure occurred prior to feeding, neither TMP-SMX nor nevirapine affected exflagellation (P > .05; Figure 6A and 6B). In contrast, lopinavir and saquinavir treatment significantly reduced exflagellation during the 72-hour period of incubation (after 24 hours, P < .01 for saquinavir at 10 µM and 5 µM; after 48 hours, P < .05 for saquinavir at 20 µM and P < .01 for lopinavir at 20 µM; after 72 hours, P < .001 for saquinavir at both 20 µM and 10 µM and P < .001 for lopinavir at both 20 µM and 10 µM). Primaquine showed significant reduction in exflagellation after 48 and 72 hours (P < .05 and P < .001, respectively; Figure 6C–E) Thus, the HIV PIs (and primaquine) decreased exflagellation over the 3-day drug exposure period, and except for saquinavir at 24 hours, this effect was more pronounced with higher concentrations.
Figure 6.
Human immunodeficiency virus (HIV) drug effect on exflagellation after prolonged parasite-drug exposure. Gametocyte maturation was assessed by testing exflagellation capacity after prolonged incubation with drug. The average number of exflagellation centers per field were counted in ≥7 fields. Exflagellation centers were counted after 24, 48, and 72 hours of incubation with drug. Pooled results from 2–4 experiments are shown. A and B, The highest concentrations for each drug tested are shown. C and D, The full range of tested concentrations of the HIV protease inhibitors lopinavir and ritonavir are shown. Lopinavir and saquinavir treatment significantly reduced exflagellation during the 72-hour period of incubation (after 24 hours, P < .01 for saquinavir at 10 µM and 5 µM; after 48 hours, P < .05 for saquinavir at 20 µM and P < .01 for lopinavir at 20 µM; after 72 hours, P < .001 for saquinavir at both 20 µM and 10 µM and P < .001 for lopinavir at both 20 µM and 10 µM). Primaquine showed significant reduction in exflagellation after 48 and 72 hours (P < .05 and P < .001, respectively; C–E).
DISCUSSION
We showed that the HIV PIs lopinavir and saquinavir, but not the NNRTI nevirapine, reduce early and late stage gametocyte viability and alter gametocyte morphology at (for lopinavir) or approaching (for saquinavir) clinically relevant treatment levels. In contrast, TMP-SMX did not reduce gametocyte viability at prophylactic levels. We also demonstrated that HIV PIs at or approaching treatment levels [11, 18, 19] reduce mosquito infectivity and impair gametocyte exflagellation under conditions of prolonged parasite-drug exposure in a dose-dependent manner. Again, lopinavir achieved all this at clinically relevant concentrations, whereas saquinavir activity occured at levels only approaching clinical relevance. Last, we showed that, at prophylactic levels, TMP-SMX does not kill gametocytes but reduces mosquito infectivity [20]. These findings underscore the potential impact that HIV treatments may have on malaria transmission and, thus, eradication efforts in the field.
HIV PIs have antimalarial effects on liver stage parasites [4] and asexual blood stage parasites, in contrast to NNRTIs, which have little or no antimalarial effect on liver and asexual blood stage parasites [10–12]. The HIV PIs lopinavir, ritonavir, saquinavir, and tipranavir have been previously shown to impair gametocytogenesis, with only tipranavir having gametocytocidal activity [9]. We have expanded this work by assessing drug effects on early and late stage gametocytes, separately, and also by assessing drug effects on transmission. We showed that early and late stage gametocytes are killed by the HIV PIs saquinavir, lopinavir, and lopinavir/ritonavir, approaching or at clinically relevant concentrations.
HIV PIs vary in potency against malaria in liver and asexual blood stage studies. Lopinavir is the most potent HIV PI, with antimalarial effect against liver and asexual blood stage parasites [4, 5], consistent with our findings for lopinavir, compared with those for saquinavir, with regard to transmission stage effects. It remains unclear why lopinavir has this activity. Interestingly, in our hands, ritonavir is ineffective at gametocyte killing, in contrast to its effect on asexual stage parasites [5]. Adding ritonavir to lopinavir-treated cultures, however, enhances the gametocyte-killing effect. Although ritonavir is typically added to boost levels of another HIV PI, the effect observed here is likely due to an additive antiparasitic effect, as lopinavir-ritonavir metabolism takes place in the liver, not the RBC [18, 19].
Our MFA data showed that lopinavir and saquinavir have dose-dependent transmission blocking activity with prolonged parasite-drug exposure, conditions that better reflect continuous administration of these drugs, as is done in clinical practice. Although the exflagellation experiments revealed that saquinavir at lower doses decreased maturation as early as day 24 at lower concentrations, the overall trend for both HIV PIs tested was a more pronounced effect of the drugs at higher doses and with more time (prolonged incubation). Because gametocytes are incubated with drug during the prolonged parasite-drug exposure prior to performance of the MFA, it is likely that the drugs are affecting parasites in the gametocyte stage more than those in the mosquito stages. This is consistent with our alamarBlue assay data, which showed that HIV PIs also kill late stage gametocytes. Additionally, and consistent with previous data, we showed that primaquine only inhibits transmission with prolonged parasite-drug exposure; because of this observation, it has been hypothesized that primaquine requires additional metabolism in the RBC for its transmission-blocking effects [8, 27]. Such an explanation also could be offered for the HIV PIs, although, again, these drugs are metabolized primarily in the liver [17–19]. It is unknown whether there are metabolites of HIV PIs or NNRTIs produced in RBCs that would affect malaria parasites.
The mechanism for the observed HIV PI effects against P. falciparum remains unclear. The proposed targets of the HIV PI in the malaria parasite are either aspartyl proteases or plasmepsins, since HIV PIs inhibit HIV aspartyl proteases [28], and investigations for HIV PI antimalarial activity have centered around this hypothesis [9, 29–31]. HIV PIs interfere with plasmepsin V and PEXEL signal sequence processing [32, 33], which is involved in export of parasite proteins to the host RBC [32]. However, this processing occurs in earlier sexual stages [34], suggesting that the HIV PI effects observed with both early and late stages could not be fully explained by interference with this process. HIV PIs could also work through other pathways or other aspartyl proteases: HIV PIs kill other Apicomplexan parasites [35], whose genomes do not encode PEXEL motifs [36]. HIV PIs could block other plasmepsins expressed in gametocytes, such as plasmepsin VI, VII, VIII, and IX [37]. Indeed, aspartyl proteases are involved in microgamete formation and in gametocyte activation [38]. Knowledge of HIV PI stage-specific activity should guide further investigation into mechanisms of action and may continue to focus on plasmepsins, which are expressed and perhaps important in these parasite stages.
Our data also show increased oocyst counts and exflagellation centers resulting from prolonged parasite-nevirapine exposure, although changes are significant in only the former. These differences could be explained if an unknown nevirapine metabolite produced within the RBC accounted for the increase in oocyst count. Also, since there was no increase in gametocytes by alamarBlue signal at day 16, the increased oocyst counts observed with nevirapine could also occur in the mosquito stages. Nevirapine could alter the mosquito gene expression, influencing patterns of parasite defenses, as chloroquine does [39]. Nevirapine could do this by altering oxidation/reduction reactions, which impact malaria parasite survival [40]. Other NNRTIs have been shown to alter oxidation/reduction reactions in mammalian cells, although the data are less clear with regard to nevirapine [41].
Separately, TMP-SMX prophylaxis has been shown to reduce clinical malaria incidence [1, 7], but its effect on transmission remains incompletely understood. In limited clinical studies, 5–7 days of TMP-SMX antimalarial treatment did not reduce gametocyte count [42, 43] or transmission [44]. However, the latter study was small and did not characterize background antifolate resistance, the existence of which dates back to this period [45]. TMP-SMX prophylaxis taken by HIV-infected patients has been associated with reduced malaria incidence in HIV-negative family members, indirectly suggesting a reduction of transmission [46]. Treatment with sulfadoxine-pyrimethamine (SP), another antifolate, has also been shown to reduce mosquito infectivity in the context of high drug efficacy [47].
Our data demonstrate that TMP-SMX does not reduce gametocyte viability at prophylactic levels in vitro: we observed an increase in the alamarBlue signal, which itself relies on oxidation/reduction reactions to generate fluorescence. Antifolates have been associated with increasing oxidative stress in animal models of malaria [48], and an increase in oxidative stress could itself theoretically have increased the signal. We also showed that TMP-SMX decreased mosquito infectivity, consistent with prior studies that tested the influence of SP, TMP, and SMX on mosquito infectivity [25, 49]. In contrast to these studies, though, we did not find that TMP-SMX affected exflagellation. However, our study differs in the timing of drug addition, drug levels, and, in the case of SP studies, possibly parasite drug sensitivity [50].
A possible limitation of our study is the differential drug effect observed in vitro versus what may occur in vivo because of protein binding. Additionally, it is possible that metabolites of these drugs may have additional effects on transmission, and this will require further study.
In conclusion, drugs used to care for HIV-infected or HIV-exposed patients have effects on malaria transmission stages in the laboratory. Clinical studies are warranted to understand the impact of these drugs on malaria transmission, given routine administration of these drugs in regions where HIV infection and malaria are prevalent. Variables such as the concentrations at which drugs have activity and antimalarial resistance patterns should be carefully assessed for their influence. Clinical assessment of the impact of HIV treatments on malaria transmission will inform optimal pharmacologic management of HIV-infected patients in malaria-endemic areas.
Notes
Acknowledgments. We thank Kevin Lee and Andre Laughinghouse, for support with mosquito rearing; Brian Kirmse and Pragyan Acharya, for critical review of this manuscript; Jeff Skinner, (Computational Biology Section, Bioinformatics and Computational Biosciences Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health [NIH]), for critical review of statistical analyses used in this manuscript; and the National Institutes of Health AIDS Research and Reference Reagent Program.
Financial support. This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Disease, National Institutes of Health; and by the US Public Health Service, National Institute of Allergy and Infectious Disease, National Institutes of Health (grant AI069314).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–56. doi: 10.1016/S1473-3099(11)70031-7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach and antiretroviral therapy for HIV infection in infants and children: towards universal access, recommendations for a public health approach. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. Accessed 1 March 2012. [PubMed] [Google Scholar]
- 3.World Health Organization. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents, and adults: recommendations for a public health approach (2006) and co-trimoxazole prophylaxis for HIV-exposed and HIV-infected infants and children: practical approaches to implementation and scale-up (2009) http://www.who.int/hiv/pub/guidelines/ctxguidelines.pdf. http://www.who.int/hiv/pub/paediatric/cotrimoxazole.pdf. Accessed 1 March 2012. [Google Scholar]
- 4.Hobbs CV, Voza T, Coppi A, et al. HIV protease inhibitors inhibit the development of preerythrocytic-stage plasmodium parasites. J Infect Dis. 2009;199:134–41. doi: 10.1086/594369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh S, Gut J, Istvan E, Goldberg DE, Havlir DV, Rosenthal PJ. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob Agents Chemother. 2005;49:2983–5. doi: 10.1128/AAC.49.7.2983-2985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Achan J, Kakuru A, Ikilezi G, et al. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med. 2012;367:2110–8. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homsy J, Dorsey G, Muhindo M, et al. Protective efficacy of prolonged trimethoprim-sulfamethoxazole prophylaxis against malaria from birth to 4 years of age among HIV-exposed children in rural Uganda (poster 1033, session 181). Program and abstracts of the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA).. 2012. Available at: http://www.retroconference.org/AbstractSearch/default2.aspx?conf=21 . Accessed 12 April 2013. [Google Scholar]
- 8.Butcher GA. Antimalarial drugs and the mosquito transmission of Plasmodium. Int J Parasitol. 1997;27:975–87. doi: 10.1016/s0020-7519(97)00079-9. [DOI] [PubMed] [Google Scholar]
- 9.Peatey CL, Andrews KT, Eickel N, et al. Antimalarial asexual stage-specific and gametocytocidal activities of HIV protease inhibitors. Antimicrob Agents Chemother. 2010;54:1334–7. doi: 10.1128/AAC.01512-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbs CV, Voza T, De La Vega P, et al. HIV nonnucleoside reverse transcriptase inhibitors and trimethoprim-sulfamethoxazole inhibit plasmodium liver stages. J Infect Dis. 2012;206:1706–14. doi: 10.1093/infdis/jis602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nsanzabana C, Rosenthal PJ. In vitro activity against Plasmodium falciparum of antiretroviral drugs. Antimicrob Agents Chemother. 2011;55:5073–7. doi: 10.1128/AAC.05130-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinner-Adams TS, McCarthy JS, Gardiner DL, Hilton PM, Andrews KT. Antiretrovirals as antimalarial agents. J Infect Dis. 2004;190:1998–2000. doi: 10.1086/425584. [DOI] [PubMed] [Google Scholar]
- 13.Sandison TG, Homsy J, Arinaitwe E, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342:d1617. doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka TQ, Williamson KC. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol Biochem Parasitol. 2011;177:160–3. doi: 10.1016/j.molbiopara.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Ellis RD, Shaffer D, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker DA. Malaria gametocytogenesis. Mol Biochem Parasitol. 2010;172:57–65. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AIDSinfo drug database. Nevirapine. http://aidsinfo.nih.gov/drugs/116/nevirapine/0/professional. Accessed 27 November 2012. [Google Scholar]
- 18.AIDSinfo drug database. Lopinavir/ritonavir. http://aidsinfo.nih.gov/drugs/316/lopinavir--ritonavir/0/professional. Accessed 12 April 2013. [Google Scholar]
- 19.AIDSinfo drug database. Saquinavir. http://aidsinfo.nih.gov/drugs/164/saquinavir/0/professional. Accessed 27 November 2012. [Google Scholar]
- 20.Zar HJ, Langdon G, Apolles P, Eley B, Hussey G, Smith P. Oral trimethoprim-sulphamethoxazole levels in stable HIV-infected children. S Afr Med J. 2006;96:627–9. [PubMed] [Google Scholar]
- 21.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher KA, Evans DA, Gilles HM, Greaves J, Bunnag D, Harinasuta T. Studies on the pharmacokinetics of primaquine. Bull World Health Organ. 1981;59:407–12. [PMC free article] [PubMed] [Google Scholar]
- 23.Mihaly GW, Ward SA, Edwards G, Orme ML, Breckenridge AM. Pharmacokinetics of primaquine in man: identification of the carboxylic acid derivative as a major plasma metabolite. Br J Clin Pharmacol. 1984;17:441–6. doi: 10.1111/j.1365-2125.1984.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Guidelines for the treatment of malaria. 2nd ed. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf . Accessed 1 March 2012. [Google Scholar]
- 25.Kone A, van de Vegte-Bolmer M, Siebelink-Stoter R, et al. Sulfadoxine-pyrimethamine impairs Plasmodium falciparum gametocyte infectivity and Anopheles mosquito survival. Int J Parasitol. 2010;40:1221–8. doi: 10.1016/j.ijpara.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21:573–80. doi: 10.1016/j.pt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Chotivanich K, Sattabongkot J, Udomsangpetch R, et al. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob Agents Chemother. 2006;50:1927–30. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dash C, Kulkarni A, Dunn B, Rao M. Aspartic peptidase inhibitors: implications in drug development. Crit Rev Biochem Mol Biol. 2003;38:89–119. doi: 10.1080/713609213. [DOI] [PubMed] [Google Scholar]
- 29.Bonilla JA, Bonilla TD, Yowell CA, Fujioka H, Dame JB. Critical roles for the digestive vacuole plasmepsins of Plasmodium falciparum in vacuolar function. Mol Microbiol. 2007;65:64–75. doi: 10.1111/j.1365-2958.2007.05768.x. [DOI] [PubMed] [Google Scholar]
- 30.Parikh S, Liu J, Sijwali P, Gut J, Goldberg DE, Rosenthal PJ. Antimalarial effects of human immunodeficiency virus type 1 protease inhibitors differ from those of the aspartic protease inhibitor pepstatin. Antimicrob Agents Chemother. 2006;50:2207–9. doi: 10.1128/AAC.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skinner-Adams TS, Andrews KT, Melville L, McCarthy J, Gardiner DL. Synergistic interactions of the antiretroviral protease inhibitors saquinavir and ritonavir with chloroquine and mefloquine against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 2007;51:759–62. doi: 10.1128/AAC.00840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boddey JA, Hodder AN, Gunther S, et al. An aspartyl protease directs malaria effector proteins to the host cell. Nature. 2010;463:627–31. doi: 10.1038/nature08728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo I, Babbitt S, Muralidharan V, Butler T, Oksman A, Goldberg DE. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature. 2010;463:632–6. doi: 10.1038/nature08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silvestrini F, Lasonder E, Olivieri A, et al. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2010;9:1437–48. doi: 10.1074/mcp.M900479-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfonso Y, Monzote L. HIV protease inhibitors: effect on the opportunistic protozoan parasites. Open Med Chem J. 2011;5:40–50. doi: 10.2174/1874104501105010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg DE, Cowman AF. Moving in and renovating: exporting proteins from Plasmodium into host erythrocytes. Nat Rev Microbiol. 2010;8:617–21. doi: 10.1038/nrmicro2420. [DOI] [PubMed] [Google Scholar]
- 37.Young JA, Fivelman QL, Blair PL, et al. The Plasmodium falciparum sexual development transcriptome: a microarray analysis using ontology-based pattern identification. Mol Biochem Parasitol. 2005;143:67–79. doi: 10.1016/j.molbiopara.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Rupp I, Bosse R, Schirmeister T, Pradel G. Effect of protease inhibitors on exflagellation in Plasmodium falciparum. Mol Biochem Parasitol. 2008;158:208–12. doi: 10.1016/j.molbiopara.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Abrantes P, Dimopoulos G, Grosso AR, do Rosario VE, Silveira H. Chloroquine mediated modulation of Anopheles gambiae gene expression. PLoS One. 2008;3:e2587. doi: 10.1371/journal.pone.0002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira Gde A, Lieberman J, Barillas-Mury C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science. 2012;335:856–9. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Sucerquia LJ, Blas-Garcia A, Marti-Cabrera M, Esplugues JV, Apostolova N. Profile of stress and toxicity gene expression in human hepatic cells treated with efavirenz. Antiviral Res. 2012;94:232–41. doi: 10.1016/j.antiviral.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Benjapongs W, Sadudi N, Noeypatimanond S. Trimethoprim-sulphamethoxazole combination in the treatment of falciparum malaria. J Med Assoc Thai. 1970;53:849–56. [PubMed] [Google Scholar]
- 43.Hansford CF, Hoyland J. An evaluation of co-trimoxazole in the treatment of Plasmodium falciparum malaria. S Afr Med J. 1982;61:512–4. [PubMed] [Google Scholar]
- 44.Wilkinson RN, Colwell EJ, Neoypatimanond S. Effect of sulphamethoxazole-trimethoprim on the viability of Plasmodium falciparum gametocytes. Trans R Soc Trop Med Hyg. 1973;67:148–9. doi: 10.1016/0035-9203(73)90340-4. [DOI] [PubMed] [Google Scholar]
- 45.Baird JK. Effectiveness of antimalarial drugs. N Engl J Med. 2005;352:1565–77. doi: 10.1056/NEJMra043207. [DOI] [PubMed] [Google Scholar]
- 46.Malamba SS, Mermin J, Reingold A, et al. Effect of cotrimoxazole prophylaxis taken by human immunodeficiency virus (HIV)-infected persons on the selection of sulfadoxine-pyrimethamine-resistant malaria parasites among HIV-uninfected household members. Am J Trop Med Hyg. 2006;75:375–80. [PubMed] [Google Scholar]
- 47.Beavogui AH, Djimde AA, Gregson A, et al. Low infectivity of Plasmodium falciparum gametocytes to Anopheles gambiae following treatment with sulfadoxine-pyrimethamine in Mali. Int J Parasitol. 2010;40:1213–20. doi: 10.1016/j.ijpara.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Legorreta-Herrera M, Retana-Ugalde R, Ventura-Gallegos JL, Narvaez V. Pyrimethamine induces oxidative stress in Plasmodium yoelii 17XL-infected mice: a novel immunomodulatory mechanism of action for an old antimalarial drug? Exp Parasitol. 2010;126:381–8. doi: 10.1016/j.exppara.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Delves M, Plouffe D, Scheurer C, et al. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med. 2012;9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersen E. In vitro susceptibility of Plasmodium falciparum malaria to pyrimethamine, sulfadoxine, trimethoprim and sulfamethoxazole, singly and in combination. Trans R Soc Trop Med Hyg. 1987;81:238–41. doi: 10.1016/0035-9203(87)90226-4. [DOI] [PubMed] [Google Scholar]