Abstract
Amphetamine was discovered over 100 years ago. Since then, it has transformed from a drug that was freely available without prescription as a panacea for a broad range of disorders into a highly restricted Controlled Drug with therapeutic applications restricted to attention deficit hyperactivity disorder (ADHD) and narcolepsy. This review describes the relationship between chemical structure and pharmacology of amphetamine and its congeners. Amphetamine’s diverse pharmacological actions translate not only into therapeutic efficacy, but also into the production of adverse events and liability for recreational abuse. Accordingly, the balance of benefit/risk is the key challenge for its clinical use. The review charts advances in pharmaceutical development from the introduction of once-daily formulations of amphetamine through to lisdexamfetamine, which is the first d-amphetamine prodrug approved for the management of ADHD in children, adolescents and adults. The unusual metabolic route for lisdexamfetamine to deliver d-amphetamine makes an important contribution to its pharmacology. How lisdexamfetamine’s distinctive pharmacokinetic/pharmacodynamic profile translates into sustained efficacy as a treatment for ADHD and its reduced potential for recreational abuse is also discussed.
Keywords: Abuse liability, amphetamine, attention deficit hyperactivity disorder (ADHD), drug formulations, lisdexamfetamine, microdialysis
A short history of amphetamine
Although racemic α-methylphenethylamine (amphetamine) was discovered by Barger and Dale in 1910, it was not until 1927 that this molecule was first synthesised by the chemist, G. A. Alles, whilst he was searching for a less costly and more easily synthesised substitute for ephedrine. Experiments performed in animals and human subjects by Alles and others unequivocally revealed α-methylphenethylamine’s ability to reverse drug-induced anaesthesia and produce arousal and insomnia (see reviews by Bett, 1946; Guttmann and Sargent, 1937). The trade name ‘Benzedrine®’ for racemic α-methylphenethylamine was registered by the pharmaceutical company, Smith, Kline and French. ‘Amphetamine’, which is the generic name for Benzedrine devised by the Council on Pharmacy and Chemistry of the American Medical Association, was not adopted until many years later. It is the reason why the name Benzedrine, not amphetamine, appears in all of the early publications (see Bett, 1946). Smith, Kline and French introduced Benzedrine onto the market in 1935 as a treatment for narcolepsy (for which it is still used today), mild depression, post-encephalitic Parkinsonism and a raft of other disorders (see Bett, 1946; Guttmann and Sargent, 1937; Tidy, 1938).
As a molecule with a single chiral centre, amphetamine exists in two optically active forms, i.e. the dextro- (or d-) and levo- (or l-) isomers or enantiomers (Figure 1). Smith, Kline and French synthesised both isomers, and in 1937 commenced marketing of d-amphetamine, which was the more potent of the two isomers, under the trade name of Dexedrine®. Sales of Benzedrine and Dexedrine in chemist stores were unrestricted until 1939, when these drugs could only be obtained either on prescription from a registered medical practitioner or by signing the Poison Register (Bett, 1946). The cognitive-enhancing properties of amphetamine were quickly recognised, with reports of Benzedrine producing improvements in intelligence tests leading to its widespread use to reduce stress and improve concentration and intellectual performance by academics, students and medical professionals (see Guttmann and Sargent, 1937; Tidy, 1938). In his 1946 review, Bett commented on the widespread use of ‘energy pills’ by the allied forces in World War II, estimating that 150 million Benzedrine tablets were supplied to British and American service personnel during the course of the global conflict. In spite of considerable coverage in the medical literature and the popular press describing the powerful central effects of these new drugs, the addictive potential of amphetamine was largely dismissed (see Bett, 1946; Guttmann and Sargent, 1937; Tidy, 1938).
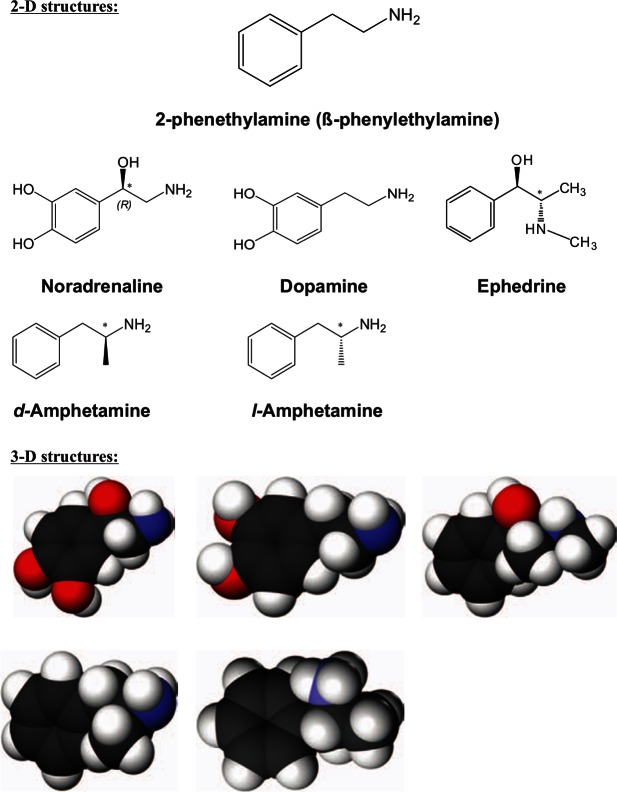
Figure 1.
Chemical structures of various biologically active β-phenylethylamines.
* Chiral centre. Red: Oxygen atom; White: Hydrogen atom; Black: Carbon atom; Blue: Nitrogen atom.
It was Bradley (1937) who first reported the beneficial effects of Benzedrine in treating children with severe behavioural problems, who would now be diagnosed as suffering from attention deficit/hyperactivity disorder (ADHD) (American Psychiatric Association, 1994). Bradley treated 30 subjects for a week, and in approximately half of them he observed remarkable improvements in their school performance, behaviour and demeanour. These therapeutic benefits unequivocally derived from the drug because they were apparent from the first day of Benzedrine treatment and disappeared as soon as it was discontinued. Although l-amphetamine (Cydril®) achieved far less attention than either the racemate or d-isomer, clinical trials conducted in the 1970s demonstrated that both isomers of amphetamine were clinically effective in treating ADHD (Arnold et al., 1972, 1973, 1976). The use of Benzedrine to treat ADHD declined dramatically after Gross (1976) reported that the racemate was significantly less clinically effective than Dexedrine. Currently, the only use of l-amphetamine in ADHD medications is in mixed salts/mixed enantiomers amphetamine (MES-amphetamine), which consists of a 3:1 enantiomeric mixture d-amphetamine:l-amphetamine salts that is available in both immediate-release (Adderall®, generic) and extended-release (Adderall XR®, generic) formulations. A recent development in the amphetamine field is the introduction of an amphetamine prodrug, lisdexamfetamine dimesylate (Vyvanse®). Lisdexamfetamine comprises the naturally occurring amino acid, L-lysine, covalently bound to d-amphetamine via an amide linking group. It has been approved for the management of ADHD in children (age 6–12), adolescents and adults in the USA and Canada. It is currently being developed for clinical use in treating ADHD in a number of European countries. The metabolic route of lisdexamfetamine is unusual because after absorption into the bloodstream it is metabolised by red blood cells to yield d-amphetamine and the natural amino acid, L-lysine, by rate- limited, enzymatic hydrolysis (Pennick, 2010). An overview of amphetamine-based medications is provided in Table 1.
Table 1.
Amphetamines – past and present.
| Product | Salt | Formulation | Trade names | Currently available |
|---|---|---|---|---|
| Racemic amphetamine | Base | IR | Benzedrine, Actedron, Allodene, Adipan, Sympatedrine, Psychedrine, Isomyn, Isoamyne, Mecodrine, Norephedrane, Novydrine, Elastonon, Ortédrine, Phenedrine, Profamina, Propisamine, Sympamine, Sympatedrin | No |
| Sulphate | IR | Benzedrine sulphate, Alentol, Psychoton, Simpamina | No | |
| Phosphate | IR | Acetemin, Aktedron, Monophos, Profetamine phosphate, Racephen, Raphetamine phosphate | No | |
| d-Amphetamine | Sulphate | IR | Dexedrine sulphate, Afatin, d-Amfetasul, Domafate Obesedrin, Dexten, Maxiton, Sympamin, Simpamina-D, Albemap, Dadex, Ardex, Dexalone, Amsustain, Betafedrina, d-Betaphedrine, Diocurb, Dextrostat, generic | Some Yes |
| Sulphate | Liquid | Procentra | Yes | |
| Sulphate | XR | Generic | Yes | |
| Tannate | IR | Synatan, Tanphetamine | No | |
| l-Amphetamine | Succinate | IR | Cydril | No |
| Mixed enantiomers/ mixed salts amphetamine (3:1 d:l isomers) |
Saccharate/ aspartate/ sulphate |
IR | Adderall, generic | Yes |
| Saccharate/ aspartate/ sulphate |
XR | Adderall XR, generic | Yes | |
| Lisdexamfetamine | Dimesylate | Prodrug | Vyvanse | Yes |
IR: immediate release; XR: extended release.
Data taken from various sources including the Merck Index, Daily Med, electronic Medicines Compendium.
A clinical perspective on the use of amphetamine in the treatment of ADHD
ADHD is arguably the most under-diagnosed and treated of all psychiatric disorders, especially in adults (Kooij et al., 2010). The most recent European data suggest that about 5% of the population suffer from ADHD in any one year, with a total of about 3 million patients in Europe (Wittchen et al., 2011). Further estimates put the cost of each patient at about £5000 per year in the UK (Gustavsson et al., 2011). Of the total just over half are direct treatment costs and the rest indirect costs, for example lost productivity, social harm, negative impact on family life, increased incidence of accidents and costs associated with criminality and legal intervention. The impact in terms of lost quality of life (days lived with disability) puts ADHD in the top 10 disorders of the brain in Europe. Treatment of ADHD is generally inadequate, with estimates suggesting that, at best, less than one-third of patients with the diagnosis get appropriate treatment (Gustavsson et al., 2011).
Although amphetamine has been established as an effective treatment for ADHD, as well as other central nervous system (CNS) disorders such as narcolepsy for decades, its use in the UK (and in the wider European context) has been rather limited in comparison with its widespread use in the USA. The reasons for this are complex and relate to social and medical attitudes to the condition of ADHD, pharmaceutical industry marketing policies, as well as to concerns regarding the use of drugs in paediatric indications which are perceived to have a high potential for recreational abuse and to cause addiction.
ADHD has long suffered from being considered an ‘American’ diagnosis, and for many decades there was a concerted attempt by some experts in child psychiatry to deny, or at least minimise, its existence in the UK. On top of this, on the rare occasions when the disorder was identified, the preferred treatment option was psychotherapy because it fitted with the background of the child psychiatrists and psychologists who were responsible for managing these patients. It was left to certain paediatricians to develop the requisite expertise in the use of stimulants for treating children with ADHD, which many did quite successfully. In recent years, child psychiatrists have begun to assume a prescribing role as well, largely using methylphenidate preparations.
Amphetamines, i.e. racemic amphetamine, d-amphetamine and methamphetamine, were widely used to promote wakefulness in World War II, which in turn led to a large increase in production that resulted in large surpluses of these drugs after the war. Much of these stocks got into the ‘black market’, and in the 1950s d-amphetamine abuse became recognised. In a classic study of that period, Connell from the Institute of Psychiatry reported a group of heavy d-amphetamine users who had become paranoid (Connell, 1966). This flagged up the potential psychiatric dangers of this drug and may have encouraged prescribers away from d-amphetamine and on to methylphenidate. Another factor was the use of d-amphetamine as an antidepressant in the 1950s before the discovery of the tricyclic monoamine reuptake inhibitors. There were cases of misuse by patients, and also a significant degree of diversion of the prescribed drug into youth misuse and/or abuse that may also have contributed to wariness by prescribers regarding its clinical use. In later years, local outbreaks of d-amphetamine abuse have occurred in various parts of the UK, often using locally synthesised d-amphetamine; again, this will have made doctors shy away from prescribing d-amphetamine lest it contributes to its misuse. In the USA, d-amphetamine-containing medications, especially MES-amphetamine, have been very widely used as treatments for ADHD. Familiarity with prescribed amphetamines together with the increased availability of more and more tamper-deterrent drug formulations to reduce the potential for abuse, for example Adderall XR®, have created a situation where in the USA the abuse risk of d-amphetamine is perceived as being similar to that of methylphenidate. This fact, along with the perception that d-amphetamine is much safer than the more potent and enduring stimulant methamphetamine, which is now widely abused, has resulted in a more relaxed attitude of physicians in the USA to the prescribing of d-amphetamine. Luckily, for reasons that are obscure, the recreational abuse methamphetamine has never really caught on in Europe, and almost all illegal use of the amphetamines is confined to d-amphetamine as the sulphate salt.
The pharmacology of amphetamine
The chemical structure, particularly the 3-dimensional (3-D) structure of amphetamine, is critical in determining the pharmacological effects that underpin its considerable therapeutic benefits and also its liability for recreational abuse. Amphetamine belongs to the class of drugs called the ‘β-phenylethylamines’. Although it was synthesised many decades before the discovery that the monoamines, i.e. noradrenaline (norepinephrine), dopamine and 5-hydroxytryptamine (5-HT; serotonin), were major neurotransmitters in the central and peripheral nervous systems, part of the rationale for synthesising racemic amphetamine was its structural similarity to the biologically active molecule, ephedrine.
As shown in Figure 1, the similarity between the chemical structures of the catecholamine neurotransmitters, noradrenaline and dopamine, and the isomers of amphetamine is abundantly clear. The 3-D structures of the catecholamines and amphetamine molecules reveal the long planar conformation that is common to all of these compounds. For amphetamine’s isomers, it is their planar conformation, molecular size that is similar to the monoamines, the presence of an aromatic ring and a nitrogen on the aryl side-chain which are the prerequisite physico-chemical properties of a competitive substrate for the monoamine reuptake transporters, i.e. NET (noradrenaline transporter), DAT (dopamine transporter) and SERT (5-HT transporter).
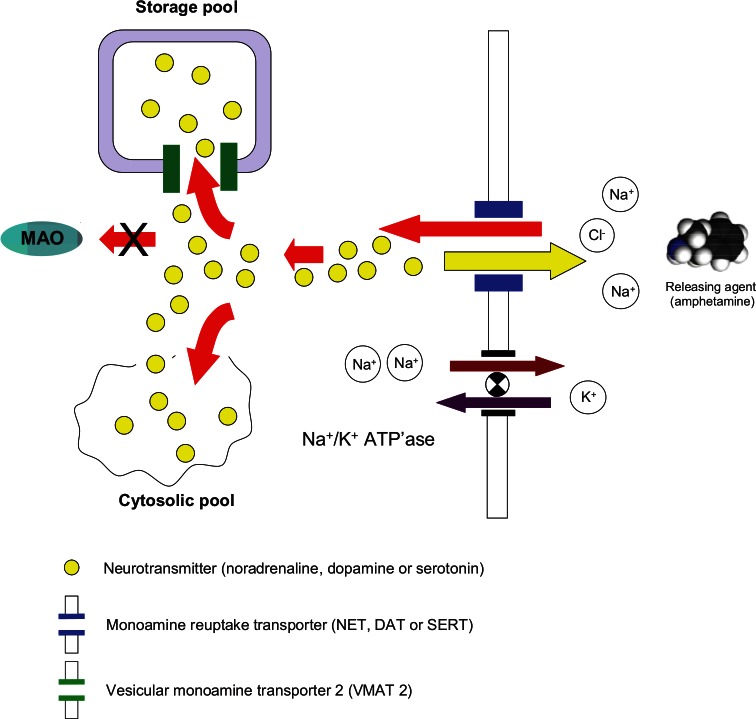
Figure 2 illustrates the mechanism responsible for the uptake transport of monoamines and amphetamine into presynaptic nerve terminals. One molecule of monoamine neurotransmitter or amphetamine associates with two Na+ and one Cl- ion, and the resulting molecular complex is actively transported into the presynaptic terminal by the relevant monoamine reuptake transporter. The motive power for this active transport mechanism is a Na+ ion concentration gradient (high Na+ on the outside of the nerve terminal/low Na+ on the inside). The Na+ concentration gradient is maintained by Na+/K+ ATPase that pumps two Na+ ions out of the cell whilst simultaneously pumping in one K+ ion. There are two pools of monoamine neurotransmitter within each type of nerve terminal: the cytosolic pool that holds newly synthesised monoamines, and the vesicular pool that stores the monoamines and from which they are released when neurones fire action potentials.
Figure 2.
Actions comprising the pharmacological mechanism of amphetamine.
Although the concentration of a monoamine neurotransmitter in the cytosol of the presynaptic nerve terminal is regulated, controlled by its rates of synthesis, release, reuptake and catabolism, it is now recognised that transport of the monoamine into the vesicular storage granules has a critically important role to play in this process. Translocation of monoamines from the cytosolic pool into the storage pool is performed by a similar active transporter system, the vesicular monoamine transporter 2 (VMAT2) (Fei et al., 2008; Fleckenstein et al., 2009; Ramamoorthy et al., 2011). Since amphetamine competes with the endogenous monoamines for transport into the nerve terminals via NET, DAT or SERT, the higher the concentration of amphetamine present in the synapse, the greater the number of amphetamine molecules transported relative to every molecule of monoamine (see Figure 3). Once inside the presynaptic terminal, amphetamine displaces monoamines from the cytosolic pool. Furthermore, because amphetamine also has affinity for VMAT2 (Teng et al., 1998), it prevents the translocation of monoamines into the intraneuronal storage vesicles. The outcome of these actions is that the direction of the reuptake transporter reverses, so that instead of pumping neurotransmitter from the synapse into the nerve terminal, it pumps neurotransmitter out of neurones into the synapse. This process is called ‘reverse transport’ or ‘retro-transport’ (Robertson et al., 2009).
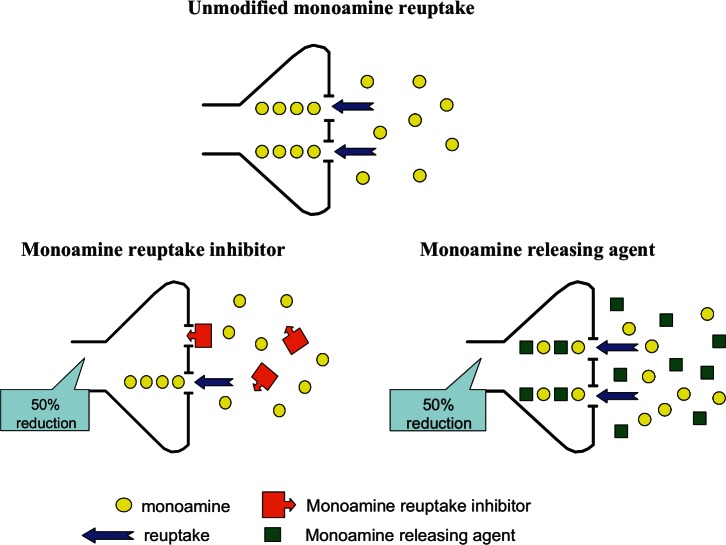
Figure 3.
Different mechanisms leading to a 50% reduction in monoamine reuptake produced by a classical reuptake inhibitor versus a competitive substrate (releasing agent).
Consistent with the mechanism described above, in vitro experiments have unequivocally demonstrated that amphetamine’s d- and l-isomers non-selectively release [3H]monoamines from preloaded slices or synaptosomes prepared from rat brain. There are experimental reports stating that d-amphetamine releases [3H] noradrenaline, dopamine and 5-HT from synaptosomes (Holmes and Rutledge, 1976; Rothman et al., 2001) and brain slices (Heal et al., 1998). l-Amphetamine releases noradrenaline, dopamine and 5-HT from synaptosomes (Heikkila et al., 1975; Holmes and Rutledge, 1976) and noradrenaline and dopamine from rat brain slices (Easton et al., 2007). Comparing the relative potencies of d- and l-amphetamine, Heikkila et al. (1975) and Easton et al. (2007) reported that the d-isomer was approximately fourfold more potent than the l-isomer as a releaser of [3H]dopamine. In contrast, l-amphetamine was either as potent, or more so, than d-amphetamine as a releaser of [3H]noradrenaline (Easton et al., 2007; Heikkila et al., 1975). The monoamine transporters are not particularly selective in terms of which monoamines they transport, and this lack of selectivity is explained by the close structural similarity between them (Figure 1). Furthermore, this structural similarity between the monoamine neurotransmitters and amphetamine explains why the latter has promiscuous actions to release the important CNS monoamines (noradrenaline, dopamine and 5-HT). Amphetamine also releases adrenaline from the peripheral sympathetic nervous system, an action linked to its cardiovascular side effects. Although most of these experiments have looked at the effects of amphetamine isomers on basal [3H]monoamine release from synaptosomes or slices, amphetamine also augments electrically stimulated efflux (Easton et al., 2007). This action indicates that its retro-transport mechanism can act both co-operatively with, and independently of, neuronal firing.
Although the pharmacological effect of amphetamine is predominantly mediated by monoamine release, this mechanism is complemented by reuptake inhibition and probably also inhibition of monoamine oxidase (MAO) that combine additively or synergistically to augment synaptic monoamine concentrations. The description of amphetamine as a ‘monoamine reuptake inhibitor’ often causes some confusion, and the difference between the mechanisms of amphetamine, which is a competitive reuptake transport substrate, and classical reuptake inhibitors is illustrated in Figure 3. The potency of amphetamine’s isomers as monoamine reuptake inhibitors is summarised in Table 2 and they are compared against some highly potent classical reuptake inhibitors. d-Amphetamine is generally accepted to be a weak dopamine reuptake inhibitor with a Ki value of ~100 nM, a moderately potent inhibitor of noradrenaline reuptake (Ki = 40–50 nM) and a very weak inhibitor of 5-HT reuptake (Ki = 1.4-3.8 µM). Comparisons of the isomers of amphetamine reveal that l-amphetamine is 3.2–7-fold less potent than d-amphetamine as a dopamine reuptake inhibitor (Easton et al., 2007; Kula and Baldessarini, 1991; Richelson and Pfenning, 1984), but it is only 1.8-fold less potent against noradrenaline (Richelson and Pfenning, 1984). Its potency is so low that l-amphetamine would not be considered to be a 5-HT reuptake inhibitor.
Table 2.
Inhibition of [3H]monoamine uptake into rat brain synaptosomes by amphetamine’s enantiomers in vitro.
| Drug | Inhibition of [3H]monoamine uptake (Ki = nM) |
Reference | ||
|---|---|---|---|---|
| [3H]Noradrenaline | [3H]Dopamine | [3H]5-HT | ||
| Amphetamine enantiomers | ||||
| d-Amphetamine | 50 39 – 45 – 55 |
82 34 225 132 78 206 |
1840 3830 – 1441 – – |
1 2 3 4 5 6 |
| l-Amphetamine | 90 – 259 |
380 720 1435 |
10,000 – – |
1 3 6 |
| Reference reuptake inhibitors | ||||
| Atomoxetine | 21 1 |
2355 1400 |
– 43 |
6 7 |
| GBR 12935 | 277 | 4 | 289 | 2 |
| Paroxetine | 33 | 1700 | 0.73 | 7 |
- = Not tested;
Finally, excess monoamines within the nerve terminal are catabolised by the mitochondrial-bound enzyme, MAO. Inhibition of MAO would further augment the quantity of neurotransmitter that is available for retro-transport into the synapse. Amphetamine’s isomers have long been known to be inhibitors of this important catabolising enzyme (Mantle et al., 1976; Miller et al., 1980; Robinson, 1985). Although this mechanism is often discounted because amphetamine is a relatively weak inhibitor of MAO, in the situation where amphetamine is concentrated in presynaptic nerve terminals, shown in Figure 3, it is probable that some inhibition of this enzyme would occur.
Although in vitro experiments provide a good insight into individual mechanisms, the efficacy of amphetamine relative to other indirect monoamine agonists, for example classical reuptake inhibitors, can only be estimated from in vivo experiments. We have used dual-probe intracerebral microdialysis to explore the in vivo effects of d- and l-amphetamine in the spontaneously hypertensive rat (SHR), which has been proposed as a rodent model of ADHD (Heal et al., 2008; Sagvolden, 2000; Sagvolden et al., 2005, 2009; see review by Wickens et al., 2011).
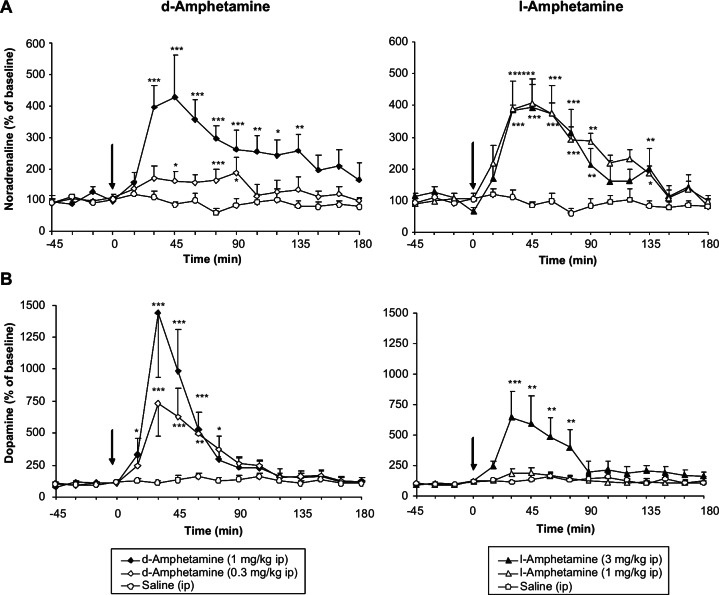
Both isomers of amphetamine dose-dependently increased the extracellular concentrations of noradrenaline in the prefrontal cortex (PFC) and dopamine in the striatum. The pharmacodynamics of their effects are typical of those reported for monoamine releasing agents, i.e. a fast onset of action with peak increases of noradrenaline and dopamine efflux occurring at 30–45 min, large effects (400–450% of baseline for noradrenaline and 700–1500% of baseline for dopamine), with a relatively rapid decline after the maximum (Figure 4). Although no comparative results have been included in this review, the magnitude of the increases produced by amphetamine’s isomers are greater than those reported for classical reuptake inhibitors such as atomoxetine or bupropion, and there is no dose-effect ceiling to amphetamine’s actions (Bymaster et al., 2002; Nomikos et al., 1989, 1990; Swanson et al., 2006; see also Heal et al., 2009, 2012). When comparing the effects of drugs on the efflux of catecholamines in the PFC it is important to take into account the highly unusual neuroanatomy of this brain region. The density of DAT sites on PFC dopaminergic neurones is very low (Hitri et al., 1991), and as a consequence, most dopamine that is released is sequestered via NET into noradrenergic neurones (Mazei et al., 2002; Morón et al., 2002; Stahl, 2003). In spite of the fact that there are few DAT sites on PFC dopaminergic neurones, their reuptake capacity is sufficient for amphetamine to evoke substantial dopamine release from them (Maisonneuve et al., 1990; Pum et al., 2007; Shoblock et al., 2003), though it has been suggested that much of the release of dopamine in the PFC comes from noradrenergic neurones (Shoblock et al., 2004).
Figure 4.
A comparison of the effects of the d- and l-isomers of amphetamine on noradrenaline and dopamine efflux in the brains of freely moving rats.
The effects of amphetamine’s d- and l-isomers on the extracellular levels of (A) noradrenaline in the prefrontal cortex and (B) dopamine in the striatum of freely moving SHRs measured by intracerebral microdialysis. Each data point represents mean % of baseline ± SEM. (n = 6–11). The vertical arrow indicates the time of administration of drug or saline. *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from appropriate control group according to ANCOVA with Williams’ test for multiple comparisons.
Data taken from Cheetham et al. (2007). Note the different doses of the two drugs.
When the in vivo pharmacological profiles of amphetamine’s isomers are compared, d-amphetamine is three to fivefold more potent than l-amphetamine (Figure 4). Moreover, an analysis of the relative effects of amphetamine’s isomers on individual catecholamines reveals d-amphetamine has greater effects on dopamine than noradrenaline, whereas l-amphetamine has a more balanced action to increase both dopaminergic than noradrenergic neurotransmission (Figure 4). Although the effects of amphetamine’s isomers shown in Figure 4 were obtained in one particular rat strain predisposed to hypertension (SHRs), similar effects of d- and l-amphetamine have been reported from experiments performed in the PFC and striatum of non-hypertensive strains (Cadoni et al., 1995; Géranton et al., 2003; Kuczenski et al., 1995; Nomikos et al., 1990; Pum et al., 2007; Figure 5). Kuczenski et al. (1995) determined the effects of both amphetamine enantiomers on caudate 5-HT release. The effect was considerably smaller than found for dopamine and there was a smaller potency separation between the two isomers.
Figure 5.
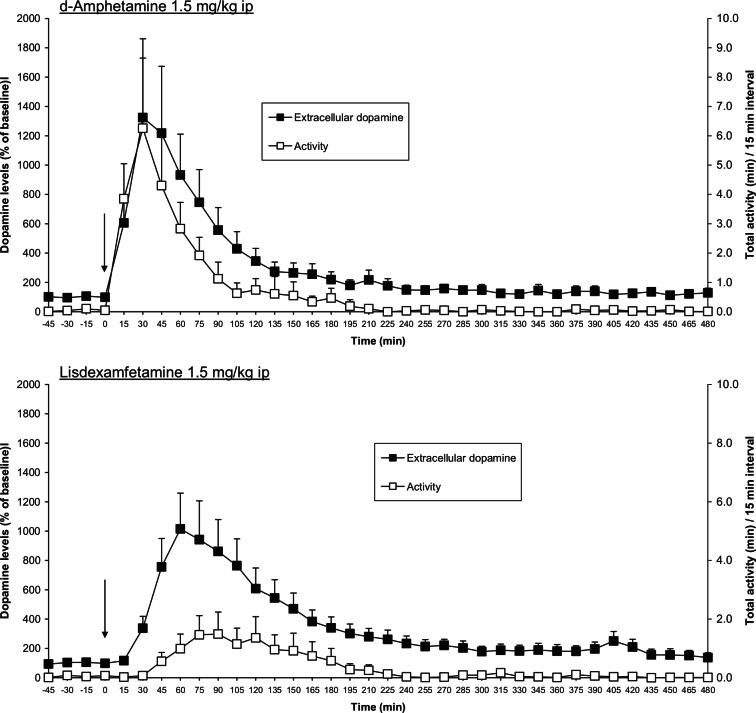
The effects of administration of d-amphetamine and lisdexamfetamine on the extracellular concentration of dopamine in the striatum and locomotor activity of freely moving rats.
Results are adjusted means; n = 5–6 ± SEM. Drug doses are expressed in terms of d-amphetamine free base for both d-amphetamine sulphate and lisdexamfetamine. The vertical arrow indicates time of drug administration. Data analysed by ANCOVA followed by multiple t-test (d-amphetamine) and Williams’ test (lisdexamfetamine). Significantly different from the vehicle-treated control group: Extracellular dopamine: d-Amphetamine (1.5 mg/kg) 0.05 > p < 0.001 at time-points 15–225 min and 255–300 min; Lisdexamfetamine (1.5 mg/kg) 0.05 > p < 0.001 at time-points 30–300 min. Activity: d-Amphetamine (1.5 mg/kg) 0.05 > p < 0.001 at time-points 15–90 min and 285–300 min; Lisdexamfetamine (1.5 mg/kg) 0.05 > p < 0.01 at time-points 60–120 min, 150–180 min and 300–315 min. Data taken from Jackson et al (2011) and Rowley et al (2011).
Earlier in the review, we described the formulation of MES-amphetamine. In vivo experiments have also been performed to explore the interaction between the 3:1 ratio of d- and l-isomers in this formulation (Glaser et al., 2005; Joyce et al., 2007). The experiments were performed in anaesthetised rats using in vivo voltammetry to determine the extracellular concentration of dopamine in the striatum and nucleus accumbens. Using this technique, Joyce et al. (2007) demonstrated that the dynamics of d-amphetamine on dopamine efflux were not altered by the presence of the l-isomer in the 1:1 ratio present in the racemate, but as the 3:1 d- to l-isomer mixture, l-amphetamine significantly enhanced and prolonged the efflux of dopamine in the rat striatum produced by d-amphetamine. The authors hypothesised that l-amphetamine in MES-amphetamine modulates the activity of DAT so that the actions of the d-isomer are prolonged (Joyce et al., 2007). An alternative explanation for the observed prolongation of pharmacological effect is that the 3:1 ratio of d- to l-isomers in the MES-amphetamine formulation is serendipitously optimised so that entry of the d-isomer into catecholaminergic nerve terminals is modulated by competition for DAT by the l-isomer, thereby prolonging the neurotransmitter-releasing action of the more potent d-isomer.
Clinical implications
The primary action of amphetamine is to increase synaptic concentrations of monoamine neurotransmitters, thereby indirectly enhancing noradrenergic, dopaminergic neurotransmission in the CNS. Although amphetamine’s isomers are also powerful 5-HT-releasing agents in vivo (Heal et al., 1998; Kuczenski et al., 1995), this action does not appear to contribute to their efficacy in treating ADHD. This opinion is based on clinical experience with fenfluramine, which is a chemical analogue of amphetamine and a powerful releasing agent with a preferential action on 5-HT (Baumann et al., 2000; Gundlah et al., 1997; Tao et al., 2002). Donnelly et al. (1989) reported that fenfluramine was not effective in treating the disruptive and overactive behaviours in ADHD; nor did it ameliorate the conduct disorder that was present in about half of the subjects. However, it is possible that the actions of amphetamine to increase serotonergic drive may have a beneficial effect on anxiety or depression that is often comorbid with ADHD. Thus, enhanced catecholaminergic signalling is the primary mediator of amphetamine’s efficacy in ADHD and narcolepsy. On the negative side, the same pharmacology is also responsible for amphetamine’s major side effects and also its liability for recreational abuse. Therefore, optimising therapeutic efficacy whilst simultaneously maintaining side effects at an acceptable level is a difficult balance requiring careful dose titration in the patient.
Efficacy
It has long been accepted that in ADHD there is dysregulation of the brain catecholaminergic systems in the PFC and its connections to subcortical regions including the striatum (Arnsten and Dudley, 2005; Durston, 2003; Russell et al., 2005). Neuroimaging studies in subjects with ADHD have revealed anatomical alterations and functional changes consistent with reduced dopaminergic function in various dopamine-rich areas of the brain including the frontal cortex, striatum and globus pallidus (Castellanos, 2001; Castellanos et al., 1996; Ernst et al., 1998; Sieg et al., 1995).
Based on observations that the isomers of amphetamine evoke very large and rapid increases in the efflux of dopamine and noradrenaline in the PFC and dopamine in the striatum, it was predicted that these drugs would be highly effective in the treatment of ADHD. This was confirmed by reports of efficacy in ADHD with d-amphetamine (Arnold et al., 1972, 1973; Gross, 1976; Huestis et al., 1975; James et al., 2001; Winsberg et al., 1974), l-amphetamine (Arnold et al., 1972, 1973), racemic amphetamine (Gross, 1976) and MES-amphetamine (Greenhill et al., 2003; James et al., 2001; Pelham et al., 1999). It is generally accepted that the efficacy of the amphetamines is not different from that of methylphenidate (Faraone et al., 2006; James et al., 2001; Pelham et al., 2005), which is the other major stimulant used to treat ADHD. However, a meta-analysis by Faraone and Buitelaar (2010) did show moderately greater efficacy for amphetamine medications. This agrees with preclinical findings that methylphenidate also markedly enhances catecholaminergic drive in the PFC and striatum (see Heal et al., 2009, 2012). On the other hand, several trials have reported the superior efficacy of amphetamine in the treatment of ADHD in comparison with the non-stimulant, selective noradrenaline reuptake inhibitor, atomoxetine (Strattera®) (Biederman et al., 2006; Faraone et al., 2007; Wigal et al., 2005). This finding fits well with results from in vivo microdialysis experiments that have shown atomoxetine can produce moderate increases in extracellular noradrenaline and dopamine in the PFC as a result of blocking the entry of both catecholamine neurotransmitters into noradrenergic neurones via NET sites, but as a selective noradrenaline reuptake inhibitor it is without effect in other brain regions, such as striatum and nucleus accumbens, where synaptic dopamine concentrations are regulated by DAT sites (Swanson et al., 2006; see also Heal et al., 2009, 2012).
Safety and adverse events
With clinical applications of amphetamine as a drug to combat fatigue, an appetite suppressant and a treatment of narcolepsy, adverse effects such as anorexia, weight loss and insomnia are predictable and frequent adverse events associated with the use of amphetamine-based medications in the management of ADHD. These side effects have been reported for d-amphetamine (James et al., 2001; Pelham et al., 1990; Winsberg et al., 1974) MES-amphetamine (Goodman et al., 2005; James et al., 2001; Pelham et al., 1999; Wigal et al., 2005) and lisdexamfetamine (Adler et al., 2008; Biederman et al., 2007a,b; Findling et al., 2008; Weisler et al., 2009). Other adverse events evoked by the amphetamines include nausea, vomiting, abdominal cramps, increases in blood pressure and heart rate and possibly also the exacerbation of motor tics (Adler et al., 2008; Biederman et al., 2007a,b; Findling et al., 2008; Goodman et al., 2005; James et al., 2001; Pelham et al., 1990, 1999; Weisler et al., 2009; Wigal et al., 2005; Winsberg et al., 1974).
Abuse liability
Stimulants have a tendency to be liked by a certain proportion of the population, though not by everyone by any means. There is some evidence that basal dopamine tone determines this, with people who have a higher number of dopamine D2 receptors as measured by [11C]-raclopride positron emission tomography (PET) finding the stimulants aversive rather than pleasurable (Volkow et al., 1999a). However, a pleasurable experience from d-amphetamine can lead to excessive use of it as a prescribed drug by the patient and the (mis)use of the prescription by others (diversion). For these reasons, all current amphetamine-type stimulant treatments are Controlled Drugs under the UK Misuse of Drugs Act 1971, with all members of being placed in Class B except methamphetamine, which was recently placed into Class A because of fears of an explosion of recreational abuse similar to that seen in the USA and Thailand.
In reality, there is little abuse of these drugs by patients with ADHD (Merkel and Kuchibhatla, 2009), and in most cases the challenge for the prescribing doctor is to keep the patients taking their medication rather than limiting its use. Many teenage patients stop using despite the drugs having clear benefits for their school performance; they cite reasons such as feeling too controlled, wanting empowerment from medication, etc. For these reasons, observations of dependence and abuse of prescription d-amphetamine are rare in clinical practice, and this stimulant can even be prescribed to people with a history of drug abuse provided certain controls, such as daily pick-ups of prescriptions, are put in place (Jasinski and Krishnan, 2009b).
It is well known that recreational drug abusers and dependent users generally administer psychostimulants at doses several-fold higher than those stipulated for therapeutic use. Furthermore, to achieve its greatest pharmacological effect, the maximum quantity of drug must be delivered into the CNS in the shortest possible time. It is this imperative which causes drug abusers to progress from relatively safe methods of self-administration, such as oral ingestion, onto increasingly dangerous routes, for example snorting cocaine, smoking (‘crack’ cocaine or ‘crystal meth’) or intravenous injection. Another less well-recognised factor in drug abuse is a desire of users for instant gratification. Thus, the appeal of a particular drug as a recreational substance of abuse is to a large extent determined by its ability to produce its desired effects within minutes, for example the cocaine ‘rush’.
The kinetics of d-amphetamine when taken orally make it less rewarding (pleasurable) than cocaine or methamphetamine. Cocaine, whether snorted or smoked as ‘crack’ in particular, enters the brain very quickly, and appears even to be concentrated in the brain relative to plasma; this explains the high rewarding potential of this drug: faster brain entry leads to a greater ‘high’. Methamphetamine enters more slowly and its peak effects are delayed by 10–15 min compared with cocaine (Fowler et al., 2008). Although d-amphetamine sulphate has not been studied in an exactly comparable way, we can predict from its physico-chemical properties that after oral ingestion d-amphetamine would have even slower rate of uptake into the brain than methamphetamine. Having said that, the abuse of d-amphetamine is not a cause for complacency. Although amphetamine abuse peaked in the 1960s (Rasmussen, 2008), the misuse of amphetamine is a persistent social, legal and medical problem (Das-Douglas et al., 2008). The intravenous use of d-amphetamine and other stimulants still pose major safety risks to the individuals indulging in this practice (Charnaud and Griffiths, 1998; Das-Douglas et al., 2008; Käll and Olin, 1990; Leino et al., 1997). Some of this intravenous abuse is derived from the diversion of ampoules of d-amphetamine, which are still occasionally prescribed in the UK for the control of severe narcolepsy and other disorders of excessive sedation. However, most intravenous d-amphetamine use is from local illicit production. Some abusers will use solvents to extract the active ingredient from tablets or capsules, which can then be concentrated and injected intravenously. The development of tamper-deterrent d-amphetamine formulations has been a major objective of the pharmaceutical industry to prevent this type of abuse. Several new once-daily d-amphetamine-containing prescription drugs have emerged that have a high degree of tamper deterrence, for example Adderall XR. In addition, lisdexamfetamine as a prodrug of d-amphetamine, is a further advance in reducing diversion risk since it provides a more gradual increase in brain drug concentration, thereby further reducing the pleasurable effects of the d-amphetamine. These topics will be revisited later in this review.
Volkow and colleagues have performed an enormous body of research using PET and other brain imaging techniques to explore the relationship between DAT occupancy, synaptic dopamine concentration and dopamine D2 receptor occupancy for psychostimulant drugs of abuse. Although the dopamine release hypothesis of drug reinforcement proposed by Di Chiara and Imperato (1988) based on experiments performed in rats and then extended in humans by Volkow and colleagues (1997, 1999a) has its limitations, it is now well accepted that euphoria, psychostimulation and reinforcement produced by stimulant drugs occur when there are rapid and substantial increases in the synaptic concentrations of dopamine in the basal striatum and mesolimbic system of the human brain. These researchers have also demonstrated that the rate of DAT occupancy by drugs such as cocaine and methylphenidate is critical to their ability to produce ‘highs’ in human subjects (Volkow and Swanson, 2003; Volkow et al., 1996a,b, 1997, 1999a,b). Although d-amphetamine is a competitive substrate for DAT rather than a classical reuptake inhibitor, these same principles apply to its pharmacological action. Thus, the rate and magnitude of neuronal dopamine release produced by amphetamine is absolutely dependent on the rate and concentration of drug that reaches DAT sites in the brain (Heal et al., 2008, 2009). There has been little research conducted in humans on this kinetic course using brain imaging, but it seems likely that the same rules apply.
Consistent with the findings in microdialysis experiments, d-amphetamine has greater potency than l-amphetamine to evoke stimulant-like subjective effects in rats (Schechter, 1978) and behavioural activation in primates (Scraggs and Ridley, 1978). Cross-generalisation occurs between the subjective cues evoked by amphetamine’s d- and l-isomers, indicating a common neurochemical mechanism (Schechter, 1978). Both amphetamine isomers have been shown to serve as positive reinforcers in animals (i.e. animals will work to get more of the drug) (Gilbert and Cooper, 1983; Risner, 1975; Yokel and Pickens, 1973). The same is true for human subjects (Smith and Davis, 1977; Van Kammen and Murphy, 1975), with the d-isomer once again being two to threefold more potent than the l-isomer (Risner, 1975; Smith and Davis, 1977; Van Kammen and Murphy, 1975; Yokel and Pickens, 1973). On the basis that the subjective and reinforcing effects of amphetamine’s isomers translate well from animals to humans, and with the assumption that the neurochemical mediators are similarly consistent across species, we can employ the findings from the microdialysis experiments to draw some conclusions on this subject. The results in Figure 4, which reveal that both isomers are equally potent noradrenaline releasers, but d-amphetamine is around threefold more potent than l-amphetamine as a dopamine releaser, point to dopamine as the primary neurochemical mediator of amphetamine’s stimulant and euphoriant properties. As indicated above, it is the combination of the rapid rate of increase and magnitude of effect that accounts for the powerful stimulant effects of amphetamine.
Although l-amphetamine is the less potent of the two isomers, its pharmacological efficacy should not be underestimated. Cheetham et al. (2007) reported that both isomers were capable of increasing striatal dopamine efflux by >5000% of baseline values, with these effects reaching a maximum within around 45 min. In contrast, the maximum increases in dopamine efflux achieved by classical dopamine reuptake inhibitors (e.g. bupropion and GBR 12909) are five to tenfold smaller, and often take longer than an hour to reach their peak (Bredeloux et al., 2007; Desai et al., 2010; Nomikos et al., 1989; Sidhpura et al., 2007; Westerink et al., 1987). The importance of the rate of increase of synaptic dopamine concentrations to the induction of stimulation and euphoria is exemplified by the observation that bupropion and GBR 12909 were not experienced as stimulant or euphoriant by normal volunteers (Hamilton et al., 1983; Peck et al., 1979; Søgaard et al., 1990) or experienced recreational stimulant users (Griffith et al., 1983; Miller and Griffith, 1983). In those bupropion and GBR 12909 trials where d-amphetamine was employed as the positive control, its stimulant, energising and reinforcing effects were unequivocally recognised by normal subjects and recreational drug users (Hamilton et al., 1983; Griffith et al., 1983; Miller and Griffith, 1983; Peck et al., 1979).
Once-daily formulations
In previous reviews, we have extensively described the efficacy and safety of stimulant and non-stimulant drugs used in the management of ADHD and compared the relative merits of each (Heal et al., 2009, 2012). This analysis has revealed that the stimulants, including amphetamine, are still accepted to be the most efficacious drugs available. Some attempts to introduce new medications, for example guanfacine XR (Intuniv®) have been successful, but many other new pharmacological approaches have failed (see Heal et al., 2012). On the other hand, the innovations in formulation technology and drug delivery systems have made significant strides forward in improving the clinical management of ADHD. All of the stimulants have biological half-lives that require at least twice-daily dosing to deliver efficacy over 12–14 h. ADHD is characterised by inattention, distractibility, working memory deficits and impulsivity, and as such, subjects with this disorder are particularly unsuited to compliance with rigid dosing schedules. Since amphetamine has a high liability for recreational abuse, placing medicines in the hands of children increases the risk of diversion/abuse, whilst the alternative approach of putting the drugs into the care of the school authorities carries with it the requirement for appropriate facilities for the storage of Controlled Drugs. Administering a once-daily stimulant medication to a child or adolescent first thing in the morning under parental supervision relieves him/her of the requirement to take additional medication outside of the home, and it also eliminates the need for the patient to take additional medication within strict time-windows. One of the additional benefits of these new formulations is their tamper deterrence, making it difficult for abusers to extract amphetamine for self-administration by hazardous routes, such as smoking, ‘snorting’ or intravenous injection. Examples of once-daily amphetamine medications include MES-amphetamine XR and the d-amphetamine prodrug, lisdexamfetamine.
Lisdexamfetamine
As briefly discussed earlier in the review, lisdexamfetamine is the first amphetamine prodrug to have been approved for use in treating ADHD. Lisdexamfetamine has no affinity for a wide panel of transporters including DAT and NET (Vyvanse®, US Product Label) or receptors, ion channels, allosteric binding sites and enzymes (Table 3). This profile is consistent with lisdexamfetamine being pharmacologically inactive. Although there is no definitive information on the subject, the large molecular size and polar characteristics of lisdexamfetamine predict that the parent molecule is unlikely to cross the blood–brain barrier. In vitro experiments revealed that the metabolism of lisdexamfetamine to d-amphetamine occurs in red blood cells by rate-limited enzymatic hydrolysis (Pennick, 2010).
Table 3.
Lack of affinity of lisdexamfetamine for a portfolio of abuse-related molecular targets
| Receptor or monoamine reuptake transporter target | |
|---|---|
| Benzodiazepine (α1 site) | Glycine (strychnine insensitive) |
| Dopamine (non-selective) | Nicotine (α-bungarotoxin insensitive) |
| GABAA | |
| GABAB | |
| Opioid (non-selective) | |
| Glutamate - AMPA - Kainate - NMDA |
Serotonin (non-selective) |
| Glycine (strychnine sensitive) | Monoamine reuptake transporter - DAT - NET - SERT |
DAT, NET, SERT = dopamine, noradrenaline and 5-HT reuptake transporters, respectively.
AMPA = 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)propanoic acid
NMDA = N-methyl-D-aspartate
GABA = γ-aminobutyric acid.
Displacement was determined in vitro at a lisdexamfetamine concentration of 10μM.
Data on file (Shire Pharmaceuticals, 2003).
The pharmacokinetic/pharmacodynamic (PK/PD) relationships of lisdexamfetamine and immediate-release (IR) d-amphetamine sulphate have been explored in rats, where automated blood sampling was combined with striatal microdialysate sampling. The locomotor activity of the rats was also simultaneously monitored. After administration of equivalent doses of lisdexamfetamine and IR d-amphetamine (1.5 mg/kg ip as d-amphetamine base), the observed plasma PK profiles for the pharmacologically active moiety, d-amphetamine, were very different. The AUC0-480 min values were identical, but the maximum concentration reached in plasma (the Cmax) was 50% lower after administration of lisdexamfetamine and the time to Cmax (the tmax) was doubled (Jackson et al., 2011). These observations are entirely consistent with the postulated rate-limited enzymatic conversion of lisdexamfetamine to d-amphetamine. This difference in PK characteristics had a profound impact on the pharmacological effects of these two compounds in rats (Figure 5). Lisdexamfetamine produced a gradual and sustained increase in striatal dopamine efflux, whereas the increase produced by IR d-amphetamine was faster in onset, reaching a peak at 30 min, and it subsequently declined more rapidly (Figure 5).
d-Amphetamine’s effects on striatal dopamine efflux and locomotor activity are superimposable (Figure 5), that is, the rapid release of dopamine translates directly into an immediate and substantial increase of locomotor activity. In the case of lisdexamfetamine, the more gradual and sustained increase in dopamine efflux was associated with a much smaller and visibly delayed locomotor response. Using a hysteresis analysis to define the relationship between the ascending and descending components of a concentration-time curve for extracellular dopamine concentration in the striatum and the functional response (locomotor activity), the relationship was anticlockwise for lisdexamfetamine, but clockwise for IR d-amphetamine (p < 0.05) (Rowley et al., 2011). Using the hysteresis analysis in a more conventional way to explore the relationship between the plasma concentration of d-amphetamine and the functional response, there was a clear difference between the two compounds with an anticlockwise hysteresis for lisdexamfetamine and no hysteresis for IR d-amphetamine (Rowley et al., 2011). The anticlockwise hysteresis shows that the functional effect of lisdexamfetamine was greater as the plasma concentration of d-amphetamine was falling, whilst the lack of hysteresis with IR d-amphetamine demonstrates that as soon as the plasma concentration of the drug starts to decline, so does its pharmacological effect.
The clinical importance of these findings will be discussed in the following section.
Implications of pharmacokinetics of lisdexamfetamine for efficacy, safety and recreational abuse liability
The efficacy of lisdexamfetamine has been demonstrated in a number of randomised, double-blind, placebo-controlled clinical trials in ADHD in children, adolescents (Biederman et al., 2007a,b; Lopez et al., 2008; Wigal et al., 2009) and adults (Adler et al., 2008, 2009; Wigal et al., 2010a). Since lisdexamfetamine has been the subject of several reviews (Dew and Kollins, 2010; Heal et al., 2009, 2012; Howland, 2008; Madaan, 2008; Mattingly, 2010; Najib, 2009), we will focus on the probable contribution of lisdexamfetamine’s special PK/PD profile to its efficacy as a treatment for ADHD and its potential for lower recreational abuse/dependence than amphetamine.
Biederman et al. (2007a) published results from the only clinical trial where the efficacy and safety of lisdexamfetamine in ADHD was compared directly against another clinically proven drug, MES-amphetamine XR. Following a 3-week, open-label run-in period where the dose of MES-amphetamine XR was optimised to 10, 20 or 30 mg once a day, subjects were then randomised into a 3-way double-blind, placebo-controlled crossover trial. They received their optimal dose of MES-amphetamine XR, an equivalent dose of lisdexamfetamine in terms of d-amphetamine base, or placebo. On the primary and secondary efficacy variables of behaviour, attention and problem solving, lisdexamfetamine delivered equivalent or better efficacy than MES-amphetamine XR with both drugs being maximally effective at 2 h post-dose (Biederman et al., 2007a). However, on the problem-solving endpoints, it was also evident that lisdexamfetamine maintained its maximum effect for at least 12 h, whereas the effect of MES-amphetamine XR showed a clear decline after 6–8 h (Biederman et al., 2007a). An exceptionally long duration of effect of lisdexamfetamine was observed by Wigal et al. (2009, 2010b), who reported that significant improvements in deportment and attention in children with ADHD were observed as early as 1 h after lisdexamfetamine administration, with its efficacy on behaviour, attention and problem solving maintained for up to 13 h. A post-hoc analysis of the data also showed that the sex and age of the subjects had no significant influence on the efficacy of lisdexamfetamine (Wigal et al., 2010b).
These observations fit well with the PD profile of lisdexamfetamine in the microdialysis experiments. Thus, a dose of lisdexamfetamine that produced only a small increase in locomotor activity evoked >500% enhancement of striatal dopamine efflux that was maintained for at least 6 h (Figure 5). PK studies in human subjects have revealed the tmax of plasma d-amphetamine occurs around 3 h after taking lisdexamfetamine; thereafter, plasma d-amphetamine declines such that at 12 h its concentration has fallen to around 60% of the Cmax (Krishnan and Stark, 2008; Krishnan et al., 2008). The maintenance of therapeutic effect in ADHD when plasma d-amphetamine concentrations are declining indicates that the anticlockwise hysteresis observed in the preclinical PK/PD experiments probably also applies to its clinical efficacy.
Another way to produce a more gentle increase of brain dopamine is to bind d-amphetamine to a support. MES-amphetamine XR employs a bead technology to deliver two bolus doses of amphetamine, the first immediately and the second approximately 4 h later, giving a Cmax for amphetamine’s d- and l-isomers 6–8 h (Adderall XR®, US Product Label). Therefore, the maximum therapeutic effect of MES-amphetamine XR (Biederman et al., 2007a) coincided almost exactly with the tmax for plasma d-amphetamine (Adderall XR®, US Product Label). These findings are also consistent with the preclinical PK/PD relationship for IR d-amphetamine that found a lack of hysteresis between plasma d-amphetamine concentration and the functional response, that is, locomotor activity.
Another factor that almost certainly contributes to the consistently high level of therapeutic efficacy observed with lisdexamfetamine treatment is the very low inter- and intra-subject variability in the plasma concentration of d-amphetamine observed after administration of the prodrug compared with traditionally formulated stimulants, including beaded and osmotic-release formulations. Once again, the reproducible pharmacokinetics of its active metabolite, d-amphetamine, are probably due to the rate-limited, enzymatic cleavage of the precursor molecule that occurs primarily in red blood cells (Ermer et al., 2010).
In two earlier published studies, Jasinski and Krishnan compared the subjective effects of lisdexamfetamine and IR d-amphetamine in drug-experienced human volunteers when these compounds were administered intravenously (Jasinski and Krishnan, 2009a) and orally (Jasinski and Krishnan, 2009b). In the trial where they compared these compounds after oral administration, IR d-amphetamine (40 mg (29.6 mg d-amphetamine base)) evoked a statistically significant increase relative to placebo in ‘Drug liking’ on the Drug Rating Questionnaire – Subject (DQRS) scale, whereas the equivalent dose of lisdexamfetamine (100 mg, oral) did not (Jasinski and Krishnan, 2009b). Furthermore, the time of lisdexamfetamine’s peak pharmacological effect was substantially delayed compared with IR d-amphetamine, at 3.0 h versus 1.5–2.0 h. When lisdexamfetamine was given at an increased dose of 150 mg, it significantly increased the DQRS ‘Drug liking’ score to an equivalent extent to IR d-amphetamine (40 mg oral). However, the peak effect of the higher dose of lisdexamfetamine was even more delayed, at 4.0 h.
When the intravenous route was explored, IR d-amphetamine (20 mg intravenous) produced a peak ‘Drug liking’ score 20 min after dosing, which coincided with plasma Cmax (Jasinsky and Krishnan, 2009b). In contrast, the equivalent dose of lisdexamfetamine (50 mg intravenous) did not significantly increase ‘Dug liking’ relative to placebo, and the Cmax of plasma d-amphetamine occurred considerably later at 2.0 h (Jasinski and Krishnan, 2009b). Both compounds yielded equivalent AUC0-24h values, but compared with the equivalent dose of IR d-amphetamine, the Cmax for plasma d-amphetamine was threefold smaller for lisdexamfetamine and the tmax was threefold greater (Jasinski and Krishnan, 2009b). These differences in the PK and PD characteristics of IR d-amphetamine and lisdexamfetamine observed in humans by Jasinski and Krishnan (2009b) are very similar to the results from the rat PK/PD study that are described earlier in this review (Jackson et al., 2011; Rowley et al., 2011).
From these results, it can be concluded that although in terms of d-amphetamine base equivalents lisdexamfetamine is clearly less potent than IR d-amphetamine, it does nonetheless produce d-amphetamine-like subjective effects in man. It is also reasonable to assume that if the intravenous dose of lisdexamfetamine had been increased, its ‘Drug liking’ effect would have separated from placebo. However, when considering any drug’s potential for recreational abuse, the time required for it to produce its peak response is likely to be as important as its magnitude. In the case of IR d-amphetamine, its maximum subjective effect occurred much earlier than lisdexamfetamine, and switching to the intravenous route speeded up IR d-amphetamine’s onset of action and increased its potency. Although increasing the dose of lisdexamfetamine enhanced its efficacy, it also progressively delayed its time of peak effect. Furthermore, switching to the intravenous route for lisdexamfetamine appeared to have relatively little influence on the abuse potential of the prodrug.
To explore this possibility further, we performed a post-hoc analysis on the data in the original clinical study reports (Jasinski, 2005, NRP104.A02; Jasinski, 2006, NRP104.A03) to compare pharmacodynamics and pharmacokinetics of lisdexamfetamine when given by the clinical route (oral) versus one of those favoured by recreational abusers (intravenous). This topic is of particular importance because lisdexamfetamine has very high aqueous solubility, making the prodrug very easy to extract. In fact, breaking the capsule open and dissolving the contents in water is stated as a dosing route for patients who are unable to swallow capsules (Vyvanse®, US Product Label).
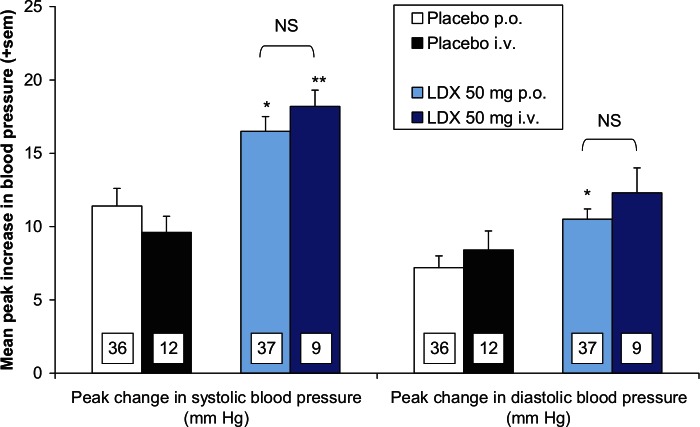
As shown in Table 4, the average maximum scores on the DQRS and Drug Rating Questionnaire – Observer (DRQO) scales for ‘Liking’, ‘Feel drug effect’, and ‘Disliking’ reveal that the subjective effects of lisdexamfetamine (50 mg) were not significantly different when the prodrug was administered orally or intravenously. This result shows that the subjective effects of lisdexamfetamine were not enhanced when the drug was given intravenously. Blood pressure measurements are useful objective measures of the PD effects of sympathomimetic drugs. Compared with placebo, 50 mg lisdexamfetamine significantly increased the peak systolic blood pressure when administered both orally and intravenously and diastolic blood pressure when given orally (Figure 6). What is also evident from the data in Figure 6 is that the magnitude of increases in systolic and diastolic blood pressures was not statistically different after oral or intravenous administration of lisdexamfetamine.
Table 4.
A comparison of the pharmacodynamics and pharmacokinetics of orally versus intravenously administered 50 mg lisdexamfetamine.
| Outcome measure (Mean or Mean ± S.D.) | LDX 50 mg, oral | LDX 50 mg, intravenous |
|---|---|---|
| Subjective effects | ||
| DRQS scales | ||
| DRQS Liking (VAS) | 2.6±0.54 | 3.1±1.67 |
| DRQS Feel drug (VAS) | 2.5±0.86 | 3.6±2.32 |
| DRQS Disliking (VAS) | 3.1±0.51 | 3.3±2.21 |
| DRQO scales | ||
| DRQO Liking (VAS) | 3.3±0.75 | 1.7±0.55 |
| DRQO Feel drug (VAS) | 2.9±0.57 | 1.9±0.65 |
| DRQO Disliking (VAS) | 1.9±0.47 | 3.0±2.0 |
| Group size | N = 36 | N = 9 |
| Pharmacokinetics | ||
| Cmax (d-amphetamine) | 41.2±11.5 | 38.9±8.1 |
| Tmax (d-amphetamine) | 4.2±1.0 | 2.5±1.5 |
| AUC0-1h (d-amphetamine) | 2.8±2.8 | 22.5±6.8 |
| AUC0-infinity (d-amphetamine) | 815±209 | 803±225 |
| Group size | N = 8 | N = 9 |
LDX: lisdexamfetamine.
Figure 6.
A comparison of the mean peak increases in systolic and diastolic blood pressure produced by intravenous versus oral administration of 50 mg lisdexamfetamine.
Means are adjusted for differences between the treatment groups at baseline. SEM was calculated from the residuals of the statistical model. Significantly different from appropriate placebo control group: *p < 0.05; **p < ;0.01. p-values for differences to compare to the same treatment by the oral and intravenous routes were obtained by the multiple t-test after fitting the data to a mixed linear model.
There were no significant differences between the peak increases in systolic and diastolic blood pressure evoked by 50 mg lisdexamfetamine administered intravenously and orally.
The PK parameters for plasma d-amphetamine observed after oral versus intravenous administration of lisdexamfetamine (50 mg) are also summarised in Table 4. The AUC0-infinity shows that the overall drug exposure was identical irrespective of the route of administration. Importantly, intravenous injection of lisdexamfetamine did not either significantly increase the Cmax of d-amphetamine, nor did it significantly reduce its tmax. Although the AUC0-1.0h indicated that early exposure to d-amphetamine was reduced after oral administration of lisdexamfetamine, this difference is probably explained by the fact that intravenous dosing route bypasses the time taken for the prodrug to be absorbed from the gut into the bloodstream prior enzymatic hydrolysis by red blood cells.
These findings strengthen the view that the unusual mechanism for metabolic conversion of lisdexamfetamine to d-amphetamine has important implications for its liability for recreational abuse. The subjective effects of a 50 mg dose of lisdexamfetamine were identical in magnitude when the prodrug was administered orally or by intravenous injection, demonstrating that intravenous injection did not enhance the pharmacological potency of lisdexamfetamine in the CNS. The increases in systolic and diastolic blood pressures after oral or by intravenous administration of lisdexamfetamine were also identical, confirming by objective and quantifiable physiological measures that the intravenous injection route did not enhance its pharmacological potency. These conclusions were supported by the PK results showing that the AUC, Cmax and tmax were not influenced by lisdexamfetamine’s route of administration.
These results are complemented by those of Ermer et al. (2011), who reported that the PK profiles were identical when lisdexamfetamine was administered intranasally or orally, indicating that attempts to increase its potential for recreational abuse by ‘snorting’ would similarly be futile. Although the findings do not demonstrate that lisdexamfetamine lacks any potential for recreational abuse, they do indicate that its attractiveness to abusers will be reduced compared with IR d-amphetamine. Based on these data, the likelihood that lisdexamfetamine will be widely abused by the intravenous or nasal route is very low.
Conclusions
It is now just over a hundred years since amphetamine was first discovered. In that period amphetamine has transformed from a drug that was widely available without prescription for the treatment of a broad range of disorders to being highly restricted Controlled Drugs that, in Europe at least, have all but disappeared from the formularies in many countries. The very clear links between molecular structure and pharmacological mode of action and, in turn, efficacy and safety in humans, makes amphetamine a textbook example of translational validity. The primary pharmacology of these drugs is not only responsible for providing efficacy in disorders such as ADHD and narcolepsy, but also for their spectrum of adverse events and liability for recreational abuse, making the balance of benefit/risk the key challenge in their clinical use. Amphetamine ranks alongside methylphenidate as the most effective drugs available for the management of ADHD, and the advances that have been made in developing genuine once-daily medications have addressed some of the problems of therapeutic coverage, whilst at the same time reducing the risk of diversion and recreational abuse.
Acknowledgments
The authors wish to thank Shire Pharmaceuticals for their support funding a portion of the writers’ time for the literature review and writing of this manuscript. The authors wish to state that the material presented in this review reflect only their views and not necessarily those of the Shire Pharmaceuticals.
Footnotes
Conflict of interest: The authors declare that there are no conflict of interest.
Funding: Part of this research was funded by Shire Pharmaceuticals.
References
- Adderall XR U.S. Product Label Available at: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=49703 (accessed August 2012).
- Adler LA, Goodman DW, Kollins SH, et al. (2008) Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. J Clin Psychiat 69: 1364–1373 [DOI] [PubMed] [Google Scholar]
- Adler LA, Weisler RH, Goodman DW, et al. (2009) Short-term effects of lisdexamfetamine dimesylate on cardiovascular parameters in a 4-week clinical trial in adults with attention-deficit/hyperactivity disorder. J Clin Psychiat 70: 1652–1661 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) The diagnostic and statistical manual of mental disorders, fourth edition. Arlington, VA: American Psychiatric Association [Google Scholar]
- Arnold LE, Huestis RD, Smeltzer DJ, et al. (1976) Levoamphetamine vs dextroamphetamine in minimal brain dysfunction. Replication, time response, and differential effect by diagnostic group and family rating. Arch Gen Psychiat 33: 292–301 [DOI] [PubMed] [Google Scholar]
- Arnold LE, Kirilcuk V, Samuel A, et al. (1973) Levoamphetamine and dextroamphetamine: Differential effect on aggression and hyperkinesis in children and dogs. Am J Psychiat 130: 165–170 [DOI] [PubMed] [Google Scholar]
- Arnold LE, Wender PH, McCloskey MM, et al. (1972) Levoamphetamine and dextroamphetamine: Comparative efficacy in hyperkinetic syndrome. Arch Gen Psychiat 27: 816–822 [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. (2005) Methylphenidate improves prefrontal cortical cognitive function through α2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, et al. (2000) Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: Therapeutic implications. Synapse 36: 102–113 [DOI] [PubMed] [Google Scholar]
- Bett WR. (1946) Benzedrine sulphate in clinical medicine: A survey of the literature. Postgrad Med J 22: 205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Boellner SW, Childress A, et al. (2007a) Lisdexamfetamine dimesylate and mixed amphetamine salts extended-release in children with ADHD: A double-blind, placebo-controlled, crossover analog classroom study. Biol Psychiat 62: 970–976 [DOI] [PubMed] [Google Scholar]
- Biederman J, Krishnan S, Zhang Y, et al. (2007b) Efficacy and tolerability of lisdexamfetamine dimesylate (NRP-104) in children with attention deficit/hyperactivity disorder: A Phase III, multicenter, randomized, double-blind, forced-dose, parallel-group study. Clin Ther 29: 450–463 [DOI] [PubMed] [Google Scholar]
- Biederman J, Wigal SB, Spencer TJ, et al. (2006) A post hoc subgroup analysis of an 18-day randomized controlled trial comparing the tolerability and efficacy of mixed amphetamine salts extended release and atomoxetine in school-age girls with attention-deficit/hyperactivity disorder. Clin Ther 28: 280–293 [DOI] [PubMed] [Google Scholar]
- Bolden-Watson C, Richelson E. (1993) Blockade by newly developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci 52: 1023–1029 [DOI] [PubMed] [Google Scholar]
- Bradley C. (1937) Behaviour of children receiving Benzedrine. Am J Psychiat 94: 577–585 [Google Scholar]
- Bredeloux P, Dubuc I, Costentin J. (2007) Comparisons between bupropion and dexamphetamine in a range of in vivo tests exploring dopaminergic transmission. Br J Pharmacol 150: 711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, et al. (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27: 699–711 [DOI] [PubMed] [Google Scholar]
- Cadoni C, Pinna A, Russi G, et al. (1995) Role of vesicular dopamine in the in vivo stimulation of striatal dopamine transmission by amphetamine: evidence from microdialysis and Fos immunohistochemistry. J Neurosci 65:1027–1039 [DOI] [PubMed] [Google Scholar]
- Castellanos FX. (2001) Neuroimaging studies of ADHD. In: Solanto MV, Arnsten AF, Castellanos FX. (eds) Stimulant drugs and ADHD: Basic and clinical neuroscience. New York: Oxford University Press, pp.243–258 [Google Scholar]
- Castellanos FX, Giedd JN, Marsh WL, et al. (1996) Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiat 53: 607–616 [DOI] [PubMed] [Google Scholar]
- Charnaud B, Griffiths V. (1998) Levels of intravenous drug misuse among clients prescribed oral dexamphetamine or oral methadone: A comparison. Drug Alcohol Depend 52: 79–84 [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Kulkarni RS, Rowley HL, et al. (2007) The SH rat model of ADHD has profoundly different catecholaminergic responses to amphetamine’s enantiomers compared with Sprague-Dawleys. Society for Neurosciences. Abstract 386.14. Available online at: www.sfn.org (accessed August 2012).
- Connell PH. (1966). Clinical manifestations and treatment of amphetamine type of dependence. JAMA 196: 718–723 [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das-Douglas M, Colfax G, Moss AR, et al. (2008). Tripling of methamphetamine/amphetamine use among homeless and marginally housed persons, 1996–2003. J Urban Health 85: 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, et al. (2010) Monoaminergic psychomotor stimulant: Discriminative stimulus effects and dopamine efflux. J Pharmacol Exp Ther 333: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew RE, Kollins SH. (2010) Lisdexamfetamine dimesylate: A new option in stimulant treatment for ADHD. Expert Opin Pharmacother 11: 2907–2913 [DOI] [PubMed] [Google Scholar]
- Donnelly M, Rapoport JL, Potter WZ, et al. (1989) Fenfluramine and dextroamphetamine treatment of childhood hyperactivity. Clinical and biochemical findings. Arch Gen Psychiat 46: 205–212 [DOI] [PubMed] [Google Scholar]
- Durston S. (2003) A review of the biological bases of ADHD: What have we learned from imaging studies? Ment Retard Dev Disabil Res Rev 9: 184–195 [DOI] [PubMed] [Google Scholar]
- Easton N, Steward C, Marshall F, et al. (2007) Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology 52: 405–414 [DOI] [PubMed] [Google Scholar]
- Ermer JC, Dennis K, Haffey MB, et al. (2011) Intranasal versus oral administration of lisdexamfetamine dimesylate: A randomized, open-label, two-period, crossover, single-dose, single-centre pharmacokinetic study in healthy adult men. Clin Drug Investig 31: 357–370 [DOI] [PubMed] [Google Scholar]
- Ermer J, Homolka R, Martin P, et al. (2010) Lisdexamfetamine dimesylate: linear dose-proportionality, low intersubject and intrasubject variability, and safety in an open-label single-dose pharmacokinetic study in healthy adult volunteers. J Clin Pharmacol 50: 1001–1010 [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, et al. (1998) DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci 18: 5901–5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Buitelaar J. (2010) Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiat 19: 353–364 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer TJ, et al. (2006) Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed 8: 4. [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Wigal SB, Hodgkins P. (2007) Forecasting three-month outcomes in a laboratory school comparison of mixed amphetamine salts extended release (Adderall XR) and atomoxetine (Strattera) in school-aged children with ADHD. J Attent Disord 11: 74–82 [DOI] [PubMed] [Google Scholar]
- Fei H, Grygoruk A, Brooks ES, et al. (2008) Trafficking of vesicular neurotransmitter transporters. Traffic 9: 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Childress AC, Krishnan S, et al. (2008) Long-term effectiveness and safety of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. CNS Spectr 13: 614–620 [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. (2009) Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: Neurotoxic and therapeutic implications. Neuropharmacology 56: 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Logan J, et al. (2008) Fast uptake and long-lasting binding of methamphetamine in the human brain – comparison with cocaine. Neuroimage 43: 756–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géranton SM, Heal DJ, Stanford SC. (2003) Differences in the mechanisms that increase noradrenaline efflux after administration of d-amphetamine: A dual-probe microdialysis study in rat frontal cortex and hypothalamus. Brit J Pharmacol 139: 1441–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D, Cooper SJ. (1983) β-Phenylethylamine-, d-amphetamine- and l-amphetamine-induced place preference conditioning in rats. Eur J Pharmacol 95: 311–314 [DOI] [PubMed] [Google Scholar]
- Glaser PE, Thomas TC, Joyce BM, et al. (2005) Differential effects of amphetamine isomers on dopamine release in the rat striatum and nucleus accumbens core. Psychopharmacology (Berl) 178: 250–258 [DOI] [PubMed] [Google Scholar]
- Goodman DW, Ginsberg L, Weisler RH, et al. (2005) An interim analysis of the quality of life, effectiveness, safety, and tolerability (QU.E.S.T.) evaluation of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr 10: 26–34 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Steinhoff K, et al. (2003) A pharmacodynamic study comparing a single morning dose of Adderall to twice-daily dosing in children with ADHD. J Am Acad Child Adolesc Psychiat 42:1234–1241 [DOI] [PubMed] [Google Scholar]
- Griffith JD, Carranza J, Griffith C, et al. (1983) Bupropion: clinical assay for amphetamine-like abuse potential. J Clin Psychiat 44: 206–208 [PubMed] [Google Scholar]
- Gross MD. (1976). A comparison of d-amphetamine and racemic-amphetamine in the treatment of the hyperkinetic syndrome or minimal brain dysfunction. Dis Nerv Sys 37: 14–16 [PubMed] [Google Scholar]
- Gundlah C, Martin KF, Heal DJ, et al. (1997) In vivo criteria to differentiate monoamine reuptake inhibitors from releasing agents: Sibutramine is a reuptake inhibitor. J Pharmacol Exp Ther 283: 581–591 [PubMed] [Google Scholar]
- Gustavsson A, Svensson M, Jacobi F, et al. ; CDBE2010Study Group (2011) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21: 718–79 [DOI] [PubMed] [Google Scholar]
- Guttmann E, Sargent W. (1937) Observations on Benzedrine. Brit Med J 1: 1013–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MJ, Smith PR, Peck AW. (1983) Effects of bupropion, nomifensine and dexamphetamine on performance, subjective feelings, autonomic variables and electro-encephalogram in healthy volunteers. Br J Clin Pharmacol 15: 367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Prow MR, et al. (1998) A comparison of the effects on central 5-HT function of sibutramine hydrochloride and other weight-modifying agents. Br J Pharmacol 125: 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Smith SL. (2009) The neuropharmacology of ADHD drugs in vivo: Insights on efficacy and safety. Neuropharmacology 57: 608–618 [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Findling RL. (2012) ADHD: Current and future therapeutics. Curr Top Behav Neurosci 9: 361–390 [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Kulkarni RS, et al. (2008) New perspectives from microdialysis studies in freely moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacol Biochem Behav 90: 184–197 [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Mytilineou C, et al. (1975) Amphetamine: Evaluation of d- and l-isomers as releasing agents and uptake inhibitors for [3H]dopamine and [3H]norepinephrine in slices of rat neostriatum and cerebral cortex. J Pharmacol Exp Ther 194: 47–56 [PubMed] [Google Scholar]
- Hitri A, Venable D, Nguyen HQ, et al. (1991) Characteristics of [3H]GBR 12935 binding in the human and rat frontal cortex. J Neurochem 56: 1663–1672 [DOI] [PubMed] [Google Scholar]
- Holmes JC, Rutledge CO. (1976) Effects of the d- and l-isomers of amphetamine on uptake, release and catabolism of norepinephrine, dopamine and 5-hydroxytryptamine in several regions of rat brain. Biochem Pharmacol 25: 447–451 [DOI] [PubMed] [Google Scholar]
- Howland RH. (2008) Lisdexamfetamine: A prodrug stimulant for ADHD. J Psychosoc Nurs Ment Health Serv 46: 19–22 [DOI] [PubMed] [Google Scholar]
- Huestis RD, Arnold LE, Smeltzer DJ. (1975) Caffeine versus methylphenidate and d-amphetamine in minimal brain dysfunction: A double-blind comparison. Am J Psychiat 132: 868–870 [DOI] [PubMed] [Google Scholar]
- Jackson HC, Rowley HL, Hackett D, et al. (2011) Comparison of the effects of equivalent doses of lisdexamfetamine dimesylate and d-amphetamine on extracellular concentrations of striatal dopamine, locomotor activity and plasma amphetamine concentrations in freely moving rats. Abstract number 775.23. Available at: http://www.SfN.org (accessed August 2012).
- James RS, Sharp WS, Bastain TM, et al. (2001) Double-blind, placebo-controlled study of single-dose amphetamine formulations in ADHD. J Am Acad Child Adolesc Psychiat 40: 1268–1276 [DOI] [PubMed] [Google Scholar]
- Jasinski D. (2005) A double-blind placebo- and active-controlled, single-dose crossover pharmacodynamic and pharmacokinetic study to evaluate the safety, tolerability and abuse liability of intravenously administered NRP104 25 mg and 50 mg in healthy adult volunteers with histories of stimulant abuse. Protocol No: NRP104.A02. Available at: http://clinicaltrials.gov/ct2/show/NCT00247572 (accessed August 2012).
- Jasinski DR. (2006) A double-blind, randomized, placebo and active-controlled, six-period crossover study to evaluate the likeability, safety, and abuse liability of NRP104 in healthy adult volunteers with histories of stimulant abuse. Protocol No: NRP104.A03. Available at: http://clinicaltrials.gov/show/NCT00248092 (accessed August 2012).
- Jasinski DR, Krishnan S. (2009a) Human pharmacology of intravenous lisdexamfetamine dimesylate: Abuse liability in adult stimulant abusers. J Psychopharmacol 23: 410–418 [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Krishnan S. (2009b) Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J Psychopharmacol 23: 419–427 [DOI] [PubMed] [Google Scholar]
- Joyce BM, Glaser PEA, Gerhardt GA. (2007) Adderall® produces increased striatal dopamine release and a prolonged time course compared to amphetamine isomers. Psychopharmacology (Berl) 191: 669–677 [DOI] [PubMed] [Google Scholar]
- Käll KI, Olin RG. (1990) HIV status and changes in risk behaviour among intravenous drug users in Stockholm 1987–1988. AIDS 4: 153–157 [DOI] [PubMed] [Google Scholar]
- Kooij SJ, Bejerot S, Blackwell A, et al. (2010) European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiat 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan SM, Stark JG. (2008) Multiple daily-dose pharmacokinetics of lisdexamfetamine dimesylate in healthy adult volunteers. Curr Med Res Opin 24: 33–40 [DOI] [PubMed] [Google Scholar]
- Krishnan SM, Pennick M, Stark JG. (2008) Metabolism, distribution and elimination of lisdexamfetamine dimesylate. Open label, single-centre, Phase I study in healthy adult volunteers. Clin Drug Invest 28: 745–755 [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, et al. (1995) Hippocampus norephinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci 15: 1308–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kula NS, Baldessarini RJ. (1991) Lack of increase in dopamine transporter binding or function in rat brain tissue after treatment with blockers of neuronal uptake of dopamine. Neuropharmacology 30: 89–92 [DOI] [PubMed] [Google Scholar]
- Leino T, Leinikki P, Hyypiä T, et al. (1997) Hepatitis: An outbreak amongst intravenous amphetamine abusers in Finland. Scand J Infect Dis 29: 213–216 [DOI] [PubMed] [Google Scholar]
- Lopez FA, Ginsberg LD, Arnold V. (2008) Effect of lisdexamfetamine dimesylate on parent-rated measures in children aged 6 to 12 years with attention-deficit/hyperactivity disorder: A secondary analysis. Postgrad Med 120: 89–102 [DOI] [PubMed] [Google Scholar]
- Madaan V. (2008) Lisdexamfetamine dimesylate for childhood ADHD. Drugs Today (Barc) 44: 319–324 [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Keller RW, Glick SD. (1990) Similar effects of d-amphetamine and cocaine on extracellular dopamine levels in medial prefrontal cortex of rats. Brain Res 535: 221–226 [DOI] [PubMed] [Google Scholar]
- Mantle TJ, Tipton KF, Garrett NJ. (1976) Inhibition of monoamine oxidase by amphetamine and related compounds. Biochem Pharmacol 25: 2073–2077 [DOI] [PubMed] [Google Scholar]
- Mattingly G. (2010) Lisdexamfetamine dimesylate: A prodrug stimulant for the treatment of ADHD in children and adults. CNS Spectr 5: 315–325 [DOI] [PubMed] [Google Scholar]
- Mazei MS, Pluto CP, Kirkbride B, et al. (2002) Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res 936: 58–67 [DOI] [PubMed] [Google Scholar]
- Merck Index, Ninth Edition (1976) Windholz M, Budavari S, Stroumtosos LY, Fertig MN. (eds) An Encyclopaedia of Chemicals and Drugs. Rahway, NJ, USA: Merck & Co., Inc [Google Scholar]
- Merkel RL, Jr, Kuchibhatla A. (2009) Safety of stimulant treatment in attention deficit hyperactivity disorder: Part I. Expert Opin Drug Saf 8: 655–668 [DOI] [PubMed] [Google Scholar]
- Miller L, Griffith J. (1983) Comparison of bupropion, dextroamphetamine, and placebo in mixed-substance abusers. Psychopharmacology 80: 199–205 [DOI] [PubMed] [Google Scholar]
- Miller HH, Shore PA, Clarke DE. (1980) In vivo monoamine oxidase inhibition by d-amphetamine. Biochem Pharmacol 29: 1347–1354 [DOI] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, et al. (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. J Neurosci 22: 389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najib J. (2009) The efficacy and safety profile of lisdexamfetamine dimesylate, a prodrug of d-amphetamine, for the treatment of attention-deficit/hyperactivity disorder in children and adults. Clin Ther 31: 142–176 [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, et al. (1989) Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology 2: 273–279 [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, et al. (1990) In vivo characterization of locally applied dopamine uptake inhibitors by striatal microdialysis. Synapse 6: 106–112 [DOI] [PubMed] [Google Scholar]
- Peck AW, Bye CE, Clubley M, et al. (1979) A comparison of bupropion hydrochloride with dexamphetamine and amitriptyline in healthy subjects. Br J Clin Pharmacol 7: 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Burrows-Maclean L, Gnagy EM, et al. (2005). Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Psychopharmacol 13: 111–126 [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Chronis AM, et al. (1999) A comparison of morning-only and morning/late afternoon Adderall to morning-only, twice-daily, and three times-daily methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 104: 1300–1311 [DOI] [PubMed] [Google Scholar]
- Pelham WE, Greenslade KE, Vodde-Hamilton M, et al. (1990) Relative efficacy of long-acting stimulants on children with attention deficit-hyperactivity disorder: A comparison of standard methylphenidate, sustained-release methylphenidate, sustained-release dextroamphetamine, and pemoline. Pediatrics 86: 226–237 [PubMed] [Google Scholar]
- Pennick M. (2010) Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat 24: 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pum M, Carey RJ, Huston JP, et al. (2007) Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology (Berl) 193: 375–390 [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. (2011) Regulation of monoamine transporters: Role of transporter phosphorylation. Pharmacol Ther 129: 220–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen N. (2008) America’s first amphetamine epidemic 1929–1971. A quantitative and qualitative retrospective with implications for the present. Am J Public Health 98: 974–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E, Pfenning M. (1984) Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: Most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol 104: 227–286 [DOI] [PubMed] [Google Scholar]
- Risner ME. (1975) Intravenous self-administration of d- and l-amphetamine by dogs. Eur J Pharmacol 32: 344–348 [DOI] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJ, Galli A. (2009) A closer look at amphetamine-induced reverse transport and trafficking of the dopamine and norepinephrine transporters. Mol Neurobiol 39: 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JB. (1985) Stereoselectivity and isoenzyme selectivity of monoamine oxidase inhibitors. Enantiomers of amphetamine, n-methylamphetamine and deprenyl. Biochem Pharmacol 34: 4105–4108 [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, et al. (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39: 32–41 [DOI] [PubMed] [Google Scholar]
- Rowley HL, Butler SA, Prow MR, et al. (2000) Comparison of the effects of sibutramine and other weight-modifying drugs on extracellular dopamine in the nucleus accumbens of freely moving rats. Synapse 38: 167–176 [DOI] [PubMed] [Google Scholar]
- Rowley HL, Cheetham SC, Hackett D, et al. (2011) Lisdexamfetamine dimesylate versus d-amfetamine – Hysteresis analyses of the relationships between extracellular striatal dopamine and locomotor activity measured in freely moving-rats. Abstract number 775.11. Available at: http://www.SfN.org (accessed August 2012).
- Russell VA, Sagvolden T, Johansen EB. (2005) Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T. (2000) Behavioural validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci Biobehav Rev 24: 31–39 [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Wøien G, et al. (2009) The spontaneously hypertensive rat model of ADHD – the importance of selecting the appropriate reference strain. Neuropharmacology 57: 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T, Russell VA, Aase H, et al. (2005) Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiat 57: 1239–1247 [DOI] [PubMed] [Google Scholar]
- Schechter MD. (1978) Stimulus properties of d-amphetamine as compared to l-amphetamine. Eur J Pharmacol 47: 461–464 [DOI] [PubMed] [Google Scholar]
- Scraggs PR, Ridley RM. (1978) Behavioural effects of amphetamine in a small primate: Relative potencies of the d- and d-isomers. Psychopharmacology (Berl) 59: 243–245 [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maisonneuve IM, Glick SD. (2004) Differential interactions of desipramine with amphetamine and methamphetamine: Evidence that amphetamine releases dopamine from noradrenergic neurons in the medial prefrontal cortex. Neurochem Res 29: 1437–1442 [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, et al. (2003) Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 165: 359–369 [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Redfern P, Rowley H, et al. (2007) Comparison of the effects of bupropion and nicotine on locomotor activation and dopamine release in vivo.Biochem Pharmacol 74: 1292–1298 [DOI] [PubMed] [Google Scholar]
- Sieg KG, Gaffney GR, Preston DF, et al. (1995) SPECT brain imaging abnormalities in attention deficit hyperactivity disorder. Clin Nucl Med 20: 55–60 [DOI] [PubMed] [Google Scholar]
- Smith RC, Davis JM. (1977) Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man. Psychopharmacology (Berl) 53: 1–12 [DOI] [PubMed] [Google Scholar]
- Søgaard U, Michalow J, Butler B, et al. (1990) A tolerance study of single and multiple dosing of the selective dopamine uptake inhibitor GBR12909 in healthy subjects. Int Clin Psychopharmacol 5: 237–251 [DOI] [PubMed] [Google Scholar]
- Stahl SM. (2003) Neurotransmission of cognition, part 1. Dopamine is a hitchhiker in frontal cortex: Norepinephrine transporters regulate dopamine. J Clin Psychiat 64: 230–231 [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Perry KW, Koch-Krueger S, et al. (2006) Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology 50: 755–760 [DOI] [PubMed] [Google Scholar]
- Tao R, Fray A, Aspley S, et al. (2002) Effects on serotonin in rat hypothalamus of d-fenfluramine, aminorex, phentermine and fluoxetine. Eur J Pharmacol 445: 69–81 [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Dwoskin LP. (1998) Lobeline displaces [3H]dihydrotetrabenazine binding and releases [3H]dopamine from rat striatal synaptic vesicles: Comparison with d-amphetamine. J Neurochem 71: 258–265 [DOI] [PubMed] [Google Scholar]
- Tidy HL. (1938) Discussion on benzedrine: Uses and abuses. Proc R Soc Med 32: 385–398 [Google Scholar]
- Van Kammen DP, Murphy DL. (1975) Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 44: 215–224 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiat 160: 1909–1918 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Foltin RW, et al. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386: 827–830 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, et al. (1996b) Relationship between psychostimulant-induced “high” and dopamine transporter occupancy. Proc Natl Acad Sci 93: 10388–10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, et al. (1999a) Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D2 receptors. J Pharmacol Exp Ther 291: 409–415 [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, et al. (1999b) Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high”. J Pharmacol Exp Ther 288: 14–20 [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Gatley SJ, et al. (1996a) Temporal relationships between the pharmacokinetics of methylphenidate in the human brain and its behavioural and cardiovascular effects. Psychopharmacology (Berl) 123: 26–33 [DOI] [PubMed] [Google Scholar]
- Vyvanse U.S. Product Label Available at: http://dailymed.nlm.nih.gov/dailymed/search.cfm?startswith=VYVANSE&x=14&y=14 (accessed August 2012).
- Weisler R, Young J, Mattingly G, et al. (2009) Long-term safety and effectiveness of lisdexamfetamine dimesylate in adults with attention deficit/hyperactivity disorder. CNS Spectr 14: 573–585 [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Damsma G, De Vries JB, et al. (1987) Dopamine reuptake inhibitors shown inconsistent effects on the in vivo release of dopamine as measured by intracerebral dialysis in the rat. Eur J Pharmacol 135: 123–128 [DOI] [PubMed] [Google Scholar]
- Wickens JR, Hyland BI, Tripp G. (2011) Animal models to guide clinical drug development in ADHD: Lost in translation? Br J Pharmacol 164: 1107–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal T, Brams M, Gasior M, et al. (2010a) Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: Novel findings using a simulated adult workplace environment design. Behav Brain Funct 6: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Kollins SH, Childress AC, et al. (2009) A 13-hour laboratory school study of lisdexamfetamine dimesylate in school-aged children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiat Ment Health 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Kollins SH, Childress AC, et al. (2010b) Efficacy and tolerability of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: Sex and age effects and effect size across the day. Child Adolesc Psychiat Ment Health 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, McGough JJ, McCracken JT, et al. (2005) A laboratory school comparison of mixed amphetamine salts extended release (Adderal XR®) and atomoxetine (Strattera®) in school-aged children with attention deficit/hyperactivity disorder. J Atten Disord 9: 275–289 [DOI] [PubMed] [Google Scholar]
- Winsberg BG, Press M, Bialer I, et al. (1974) Dextroamphetamine and methylphenidate in the treatment of hyperactive/aggressive children. Pediatrics 53: 236–241 [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, et al. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21: 655–679 [DOI] [PubMed] [Google Scholar]
- Yokel RA, Pickens R. (1973) Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther 187: 27–33 [PubMed] [Google Scholar]