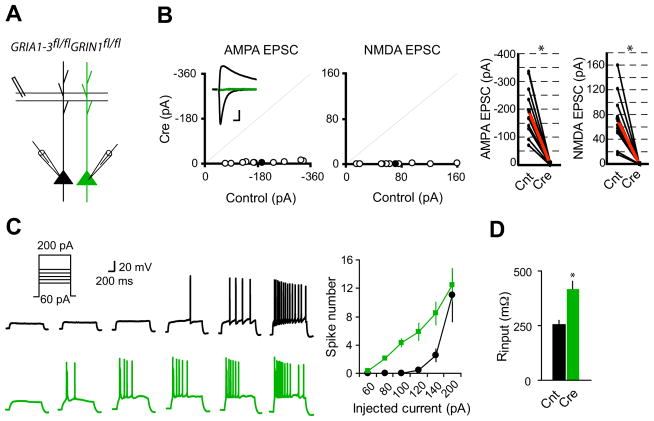
Figure 1. Single-cell synaptic silencing in vivo induced enhanced neuronal excitability.
(A) Schematic of dual whole-cell voltage-clamp recording in acute hippocampal slices from GRIA1-3fl/flGRIN1fl/fl p20-p28 mice infected with virus expressing CreGFP at p0.
(B) Scatter plots showed amplitudes of EPSCs for single pairs (open circles) and mean ± SEM (filled circles). (Inset) Representative superimposed traces (Black, Control (Cnt); Green, Cre). Scale bar: 0.02 s, 50 pA. Graphs in the right show that synaptic transmission mediated by AMPARs or NMDARs is essentially eliminated in CreGFP-expressing neurons (each black line represents a single pair and the red line represents the average; AMPA EPSCs, Cnt: −194.2 ± 29.8 pA; Cre: −4.92 ± 1.39 pA; n = 10; NMDA EPSCS, Cnt: 71.5 ± 14.03 pA; Cre: 0.56 ± 0.08 pA; n = 10). *P < 0.001.
(C) Sample action potential responses to step current injections (60, 80, 100, 120, 140 and 200 pA; 1000 ms) in control neurons (black) or CreGFP-expressing neurons (Green). Right is the summary graph showing that deletion of both AMPARs and NMDARs increased the excitability of CA1 pyramidal neurons. *P < 0.001.
(D) Bar graph shows that input resistance is significantly enhanced in neurons lacking both AMPARs and NMDARs. *P < 0.001.